ASTM F366-24
(Specification)Standard Specification for Fixation Pins and Wires
Standard Specification for Fixation Pins and Wires
ABSTRACT
This specification covers the material requirements and functional dimensions for fixation pins and wires. Factors such as bending strength, fatigue strength, break strength (Knowles type only), torsion strength, and ductility are considered important but the values and associated test methods for which have not been established yet.
SCOPE
1.1 This specification covers functional dimensions for fixation pins and wires.
1.2 In recognition of many broad and varied uses of such pins and wires, many options are included. A variety, but not necessarily all, of the options are illustrated in Figs. 1-3.
FIG. 1 Fixation Pins and Wires
Note 1: Pins and wires may be smooth shank or threaded.
Note 2: Point angle and helix angle, where applicable, is as specified by the manufacturer.
Note 3: On square or triangular shanks, flats are equal and corners are on the same circumference as the pin diameter. Shank diameters on pins larger than 3.2 mm may be reduced.
Note 4: Optional designs, both ends pointed or point with suture hole.
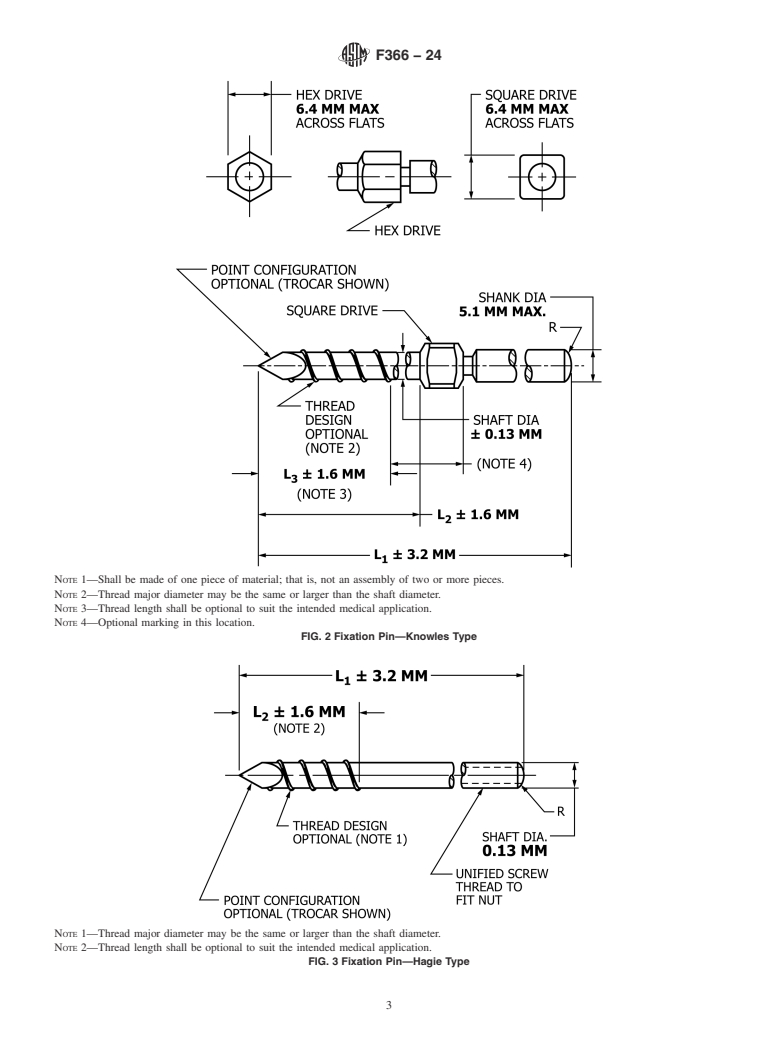
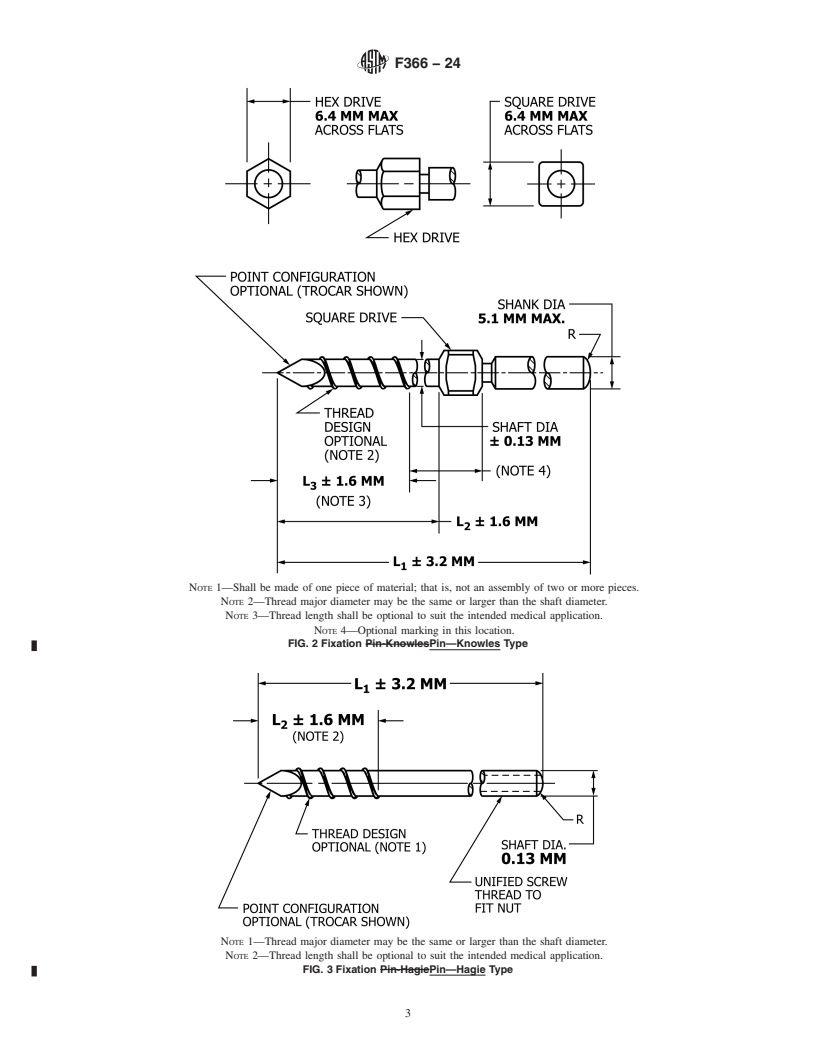
FIG. 2 Fixation Pin—Knowles Type
Note 1: Shall be made of one piece of material; that is, not an assembly of two or more pieces.
Note 2: Thread major diameter may be the same or larger than the shaft diameter.
Note 3: Thread length shall be optional to suit the intended medical application.
Note 4: Optional marking in this location.
FIG. 3 Fixation Pin—Hagie Type
Note 1: Thread major diameter may be the same or larger than the shaft diameter.
Note 2: Thread length shall be optional to suit the intended medical application.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F366 − 24
Standard Specification for
1
Fixation Pins and Wires
This standard is issued under the fixed designation F366; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope* 3. Materials
1.1 This specification covers functional dimensions for 3.1 Fixation pins shall be fabricated from a metallic material
fixation pins and wires. intended for surgical implant applications. In addition, the
materials shall be biocompatible for the intended application.
1.2 In recognition of many broad and varied uses of such
Materials should be chosen based on the design requirements
pins and wires, many options are included. A variety, but not
of the particular device. ASTM Subcommittee F04.12 main-
necessarily all, of the options are illustrated in Figs. 1-3.
tains a number of specifications for materials that are suitable
1.3 The values stated in SI units are to be regarded as
for surgical implant applications.
standard. No other units of measurement are included in this
4. Performance Requirements
standard.
4.1 Factors considered to be important, but for which values
1.4 This standard does not purport to address all of the
and test methods have not been established, are bending
safety concerns, if any, associated with its use. It is the
strength, fatigue strength, breaking strength (Knowles type
responsibility of the user of this standard to establish appro-
only), torsion strength, and ductility.
priate safety, health, and environmental practices and deter-
mine the applicability of regulatory limitations prior to use.
5. Dimensions and Characteristics
1.5 This international standard was developed in accor-
5.1 Fixation pins and wires shall be fabricated in accordance
dance with internationally recognized principles on standard-
with the dimensions illustrated in Figs. 1-4.
ization established in the Decision on Principles for the
Development of International Standards, Guides and Recom-
5.2 Fixation pins and wires shall have surfaces prepared and
mendations issued by the World Trade Organization Technical
marked in accordance with Practice F86.
Barriers to Trade (TBT) Committee.
5.2.1 Optional marking on the fixation pins and wires shall
identify the manufacturer or distributor.
2. Referenced Documents
6. Packaging and Labeling
2
2.1 ASTM Standards:
6.1 Packaging shall be adequate to protect the fixation pins
F86 Practice for Surface Preparation and Marking of Metal-
and wires during shipment.
lic Surgical Implants
F368 Specification for Fixation Pins-Knowles and Hagie
6.2 Labeling for fixation pins and wires shall include:
3
Types (Withdrawn 1982)
6.2.1 Product name,
F2503 Practice for Marking Medical Devices and Other
6.2.2 Size, on the immediate container,
Items for Safety in the Magnetic Resonance Environment
6.2.2.1 Length,
6.2.2.2 Diameter (if round) or cross-sectional size (if square
or hexagonal), that is, 6.4 mm square, and
1
This specification is under the jurisdiction of ASTM Committee F04 on
6.2.3 ASTM material specification designation number.
Medical and Surgical Materials and Devices and is the direct responsibility of
Subcommittee F04.21 on Osteosynthesis.
6.3 Consider Practice F2503 to identify potential hazards
Current edition approved Jan. 1, 2024. Published January 2024. Originally
produced by interactions between the device and the MR
approved in 1973. Last previous edition approved in 2017 as F366 – 17. DOI:
environment and for terms that may be used to label the device
10.1520/F0366-24.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
for safety in the MR environment.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on 7. Keywords
the ASTM website.
3 7.1 fixation materials; flexible surgical wire; orthopaedic
The last approved version of this historical standard is referenced on
www.astm.org. medical devices; wire-surgical implants
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F366 − 24
NOTE 1—Pins and wires may be smooth shank or threaded.
NOTE 2—Point angle and helix angle, where applicable, is as specified by the manufacturer.
NOTE 3—On square or triangular shanks, flats are equal and corners are on the same ci
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F366 − 17 F366 − 24
Standard Specification for
1
Fixation Pins and Wires
This standard is issued under the fixed designation F366; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope Scope*
1.1 This specification covers functional dimensions for fixation pins and wires.
1.2 In recognition of many broad and varied uses of such pins and wires, many options are included. A variety, but not necessarily
all, of the options are illustrated in Figs. 1-3.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of
regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
F86 Practice for Surface Preparation and Marking of Metallic Surgical Implants
3
F368 Specification for Fixation Pins-Knowles and Hagie Types (Withdrawn 1982)
F2503 Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment
3. Materials
3.1 Fixation pins shall be fabricated from a metallic material intended for surgical implant applications. In addition, the materials
shall be biocompatible for the intended application. Materials should be chosen based on the design requirements of the particular
device. ASTM committeeSubcommittee F04.12 maintains a number of specifications for materials that are suitable for surgical
implant applications.
4. Performance Requirements
4.1 Factors considered to be important, but for which values and test methods have not been established, are bending strength,
fatigue strength, breaking strength (Knowles Typetype only), torsion strength, and ductility.
1
This specification is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.21 on Osteosynthesis.
Current edition approved Sept. 1, 2017Jan. 1, 2024. Published October 2017January 2024. Originally approved in 1973. Last previous edition approved in 20102017 as
F366 – 10 (2015).F366 – 17. DOI: 10.1520/F0366-17.10.1520/F0366-24.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
The last approved version of this historical standard is referenced on www.astm.org.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F366 − 24
NOTE 1—Pins and wires may be smooth shank or threaded.
NOTE 2—Point angle and helix angle, where applicable, is as specified by the manufacturer.
NOTE 3—On square or triangular shanks, flats are equal and corners are on the same circumference as the pin diameter. Shank diameters on pins larger
than 3.2 mm may be reduced.
NOTE 4—Optional designs, both ends pointed or point with suture hole.
FIG. 1 Fixation Pins and Wires
5. Dimensions and Characteristics
5.1 Fixation pins and wires shall be fabricated in accordance with the dimensions illustrated in Figs. 1-4.
5.2 Fixation pins and wires shall have surfaces prepared and marked in accordance with Practice F86.
5.2.1 Optional marking on the fixation pins and wires shall identify the manufacturer or distributor.
6. Packaging and Labeling
6.1 Packaging shall be adequate to protect the fixation pins and wires during shipment.
6.2 Labeling for fixation pins and wires shall include:
6.2.1 Product name,
2
---------------------- Page: 2 ----------------------
F366 − 24
NOTE 1—Shall be made of one piece of material; that is, not an assembly
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.