ASTM D1016-99(2004)e1
(Test Method)Standard Test Method for Purity of Hydrocarbons from Freezing Points
Standard Test Method for Purity of Hydrocarbons from Freezing Points
SCOPE
1.1 This test method covers the sampling and determination of purity of essentially pure compounds for which the freezing points for zero impurity and cryoscopic constants are given. The compounds to which the test method is applicable are: ( Warning-Extremely flammable liquids and liquefied gases.)n-butane1,3-butadieneisobutaneisoprene(2-methyl-1,3-butadiene)n-pentanebenzeneisopentanetoluene (methylbenzene)n-hexaneethylbenzenen-heptaneo-xylene (1,2-dimethylbenzene)n-octanem-xylene (1,3-dimethylbenzene)2,2,4-trimethylpentanep-xylene (1,4-dimethylbenzene)methylcyclohexanestyrene (ethenylbenzene)isobutene
1.2 The values stated in SI units are to be regarded as the standard. The values in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see Sections 1, 6, 8, and 10-26.
Note 1 -- This test method covers systems in which the impurities form with the major component a substantially ideal or sufficiently dilute solution, and also systems which deviate from the ideal laws, provided that, in the latter case, the lowering of the freezing point as a function of the concentration is known for each most probable impurity in the given substance.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
e1
Designation:D1016–99(Reapproved 2004)
Standard Test Method for
Purity of Hydrocarbons from Freezing Points
This standard is issued under the fixed designation D1016; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—Warning notes were editorially moved into the standard text in May 2004.
1. Scope 2. Referenced Documents
1.1 This test method covers the sampling and determination 2.1 ASTM Standards:
of purity of essentially pure compounds for which the freezing D1015 Test Method for Freezing Points of High-Purity
points for zero impurity and cryoscopic constants are given. Hydrocarbons
The compounds to which the test method is applicable are:
3. Summary of Test Method
(Warning—Extremelyflammableliquidsandliquefiedgases.)
3.1 After measurement of the freezing point of the actual
n-butane 1,3-butadiene
isobutane isoprene(2-methyl-1,3-butadiene)
sample, purity can be calculated from the value of the
n-pentane benzene
determinedfreezingpointandthevaluesgivenforthefreezing
isopentane toluene (methylbenzene)
point for zero impurity and for the applicable cryoscopic
n-hexane ethylbenzene
n-heptane o-xylene (1,2-dimethylbenzene)
constant or constants.
n-octane m-xylene (1,3-dimethylbenzene)
3.2 For the equilibrium between an infinitesimal amount of
2,2,4-trimethylpentane p-xylene (1,4-dimethylbenzene)
thecrystallinephaseofthemajorcomponentandaliquidphase
methylcyclohexane styrene (ethenylbenzene)
isobutene
ofthemajorcomponentandoneormoreothercomponents,the
thermodynamic relation between the temperature of equilib-
1.2 The values stated in SI units are to be regarded as the
rium and the composition of the liquid phase is expressed by
standard. The values in parentheses are for information only.
the equation:
1.3 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
21nN 521n ~1 2N ! 5A~t 2t !@1 1B~t 2t ! 1.] (1)
1 2 f0 f f0 f
responsibility of the user of this standard to establish appro-
where:
priate safety and health practices and determine the applica-
N = mole fraction of the major component,
bility of regulatory limitations prior to use. For specific hazard
N =(1−N )=sum of the mole fractions of all the other
2 1
statements, see Sections 1, 6, 8, and 10-26.
components,
NOTE 1—Thistestmethodcoverssystemsinwhichtheimpuritiesform
with the major component a substantially ideal or sufficiently dilute
solution, and also systems which deviate from the ideal laws, provided
that, in the latter case, the lowering of the freezing point as a function of
the concentration is known for each most probable impurity in the given
substance.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
This test method is under the jurisdiction of ASTM Committee D02 on the ASTM website.
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee For a more complete discussion of this test method, see Glasgow, A. R., Jr.,
D02.04 on Hydrocarbon Analysis. Streiff, A. J., and Rossini, F. D., “Determination of the Purity of Hydrocarbons by
Current edition approved May 1, 2004. Published May 2004. Originally Measurement of Freezing Points,” Journal of Research, JRNBA, National Institute
approved in 1949. Last previous edition approved in 1999 as D1016–99. of Standards and Technology, Vol 35, No. 6, 1945, p. 355.
2 5
Numerical constants in this test method were taken from the most recently For details, see Taylor, W. J., and Rossini, F. D., “Theoretical Analysis of
published data appearing in “Tables of Physical and Thermodynamic Properties of Time-Temperature Freezing and Melting Curves as Applied to Hydrocarbons,”
Hydrocarbons and Related Compounds,” or ASTM DS 4A, Physical Constants of Journal of Research, JRNBA, Nat. Bureau Standards, Vol 32, No. 5, 1944, p. 197;
Hydrocarbons C to C , or both, prepared by the American Petroleum Institute, also Lewis, G. N., and Randall, M., “Thermodynamics and the Free Energy of
1 10
Research Project 44. ChemicalSubstances,”1923,pp.237,238,McGraw-HillBookCo.,NewYork,NY.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
e1
D1016–99 (2004)
death. Contact can cause skin irritation and dermatitis. Use
t = freezing point, in degrees Celsius, of the given
f
refrigerant bath only with adequate ventilation!)
substance (in which the mole fraction of the major
6.2 Liquid Nitrogen or Liquid Air—(Warning—Extremely
component is N ), defined as the temperature at
cold. Liberates gas which can cause suffocation. Contact with
which an infinitesimal amount of crystals of the
skin causes burns or freezing, or both. Vapors can react
major component is in thermodynamic equilibrium
violently with hot magnesium or aluminum alloys.) For use as
with the liquid phase (see Note 3 of Test Method
a refrigerant. If obtainable, liquid nitrogen is preferable be-
D1015),
cause of its safety.
t = freezing point for zero impurity, in degrees Celsius,
f0
6.2.1 Use liquid nitrogen refrigerant only with adequate
for the major component when pure, that is, when
ventilation. If liquid air is used as a refrigerant, it is imperative
N =1or N =0,
1 2
that any glass vessel containing hydrocarbon or other combus-
A = first or main cryoscopic constant, in mole fraction
per degree, and tible compound and immersed in liquid air be protected with a
B = secondary cryoscopic constant, in mole fraction per suitable metal shield. The mixing of a hydrocarbon or other
degree.
combustible compound with liquid air due to the breaking of a
Neglectingthehighertermsnotwritteninthebrackets,Eq1 glass container would almost certainly result in a violent
can be transformed to the equation: explosion. If liquid nitrogen is used as a refrigerant, no
hydrocarbonsampleshouldeverbepermittedtocoolbelowthe
log P 52.00000 2 A/2.3026! t 2t ! 1 1B t 2t ! (2)
~ ~ @ ~ #
10 f0 f f0 f
condensation temperature of oxygen (−183°C at atm). This
where:
would not be likely to occur in normal operation, but might
P = purity of the given substance in terms of mole percent
occur if the apparatus were left unattended for some time.
of the major component.
7. Procedure
4. Significance and Use
7.1 Measure the freezing point as described in Test Method
D1015, using the modifications and constants given in Sec-
4.1 The experimental procedures and physical constants
tions8-26ofthistestmethodforthespecificcompoundsbeing
provided by this test method, when used in conjunction with
examined.
Test Method D1015, allow the determination of the purity of
the material under test. A knowledge of the purity of these
NOTE 2—Theestimateduncertaintyinthecalculatedvalueofthepurity
hydrocarbonsisoftenneededtohelpcontroltheirmanufacture
as referred to in Sections 8-26 is not equivalent to the precision defined in
and to determine their suitability for use as reagent chemicals RR D02-1007.
or for conversion to other chemical intermediates or finished
8. n-Butane (Warning—Extremely flammable liquefied
products.
gas under pressure. Vapor reduces oxygen available for
breathing.)
5. Apparatus
8.1 Determine the freezing point from freezing curves, with
5.1 Sampling Apparatus, as shown in Fig. 1, for withdraw-
thecagestirrer,withacoolingbathofliquidnitrogen(orliquid
ing liquefied gases (for example, 1,3-butadiene) from pressure
air), with a cooling rate of 0.3 to 0.8°C/min for the liquid near
storage cylinders.
the freezing point, and with crystallization induced immedi-
5.2 Distilling Apparatus, as shown in Fig. 2, for removing
ately below the freezing point by means of a cold rod.
small amounts of polymer from low-boiling compounds (for
8.2 The method of obtaining the samples shall be as
example, 1,3-butadiene) by simple distillation at atmospheric
follows: Assemble the apparatus for obtaining the sample as
pressure.
shown in Fig. 1, but with no lubricant on the ground-glass
5.3 Distilling Apparatus, as shown in Fig. 3, for removing
joints and with the valve at the bottom of the cylinder, so that
smallamountsofpolymerfromcompoundswithboilingpoints
sampling is from the liquid phase. Attach to C an absorption
near room temperature (for example, isoprene) by distillation
tube containing anhydrous calcium sulfate or other suitable
at atmospheric pressure.
desiccant (except magnesium perchlorate) so that water is not
5.4 Vacuum Distilling Apparatus and Transfer Trap,as
introduced into the system (Note 3). Fill the flask F with the
shown in Fig. 4, for removing dissolved air and large amounts
carbon dioxide refrigerant to within about 51 mm (2 in.) of the
of polymer from a compound (for example, 1,3-butadiene or
top. After about 20 or 30 min, when the system will have
styrene), by repeated freezing and evacuation, followed by
cooled sufficiently, remove the absorption tube and begin the
distillation of the compound in vacuum in a closed system.
collection of liquid n-butane by opening the valve K and
adjusting the needle valve J so that the sample is collected at
6. Materials
a rate of 1 to 2 mL (liquid)/min in the condensing tube E.
6.1 Carbon Dioxide Refrigerant—Solid carbon dioxide in a
NOTE 3—However,ifsomewaterdoescondensewiththehydrocarbon,
suitable liquid. (Warning—Extremely cold (−78.5°C). Liber-
the freezing point will not be affected significantly because of the
ates heavy gas which can cause suffocation. Contact with skin
causes burns or freezing, or both. Vapors can react violently
with hot magnesium or aluminum alloys.) Acetone is
For further details, see Glasgow, A. R., Jr., et al. “Determination of Purity by
recommended.(Warning—Extremely flammable. Harmful if
Measurement of Freezing Points of Compounds Involved in the Production of
inhaled. High concentrations can cause unconsciousness or Synthetic Rubber,” Analytical Chemistry, ANCHA, Vol 20, 1948, p. 410.
e1
D1016–99 (2004)
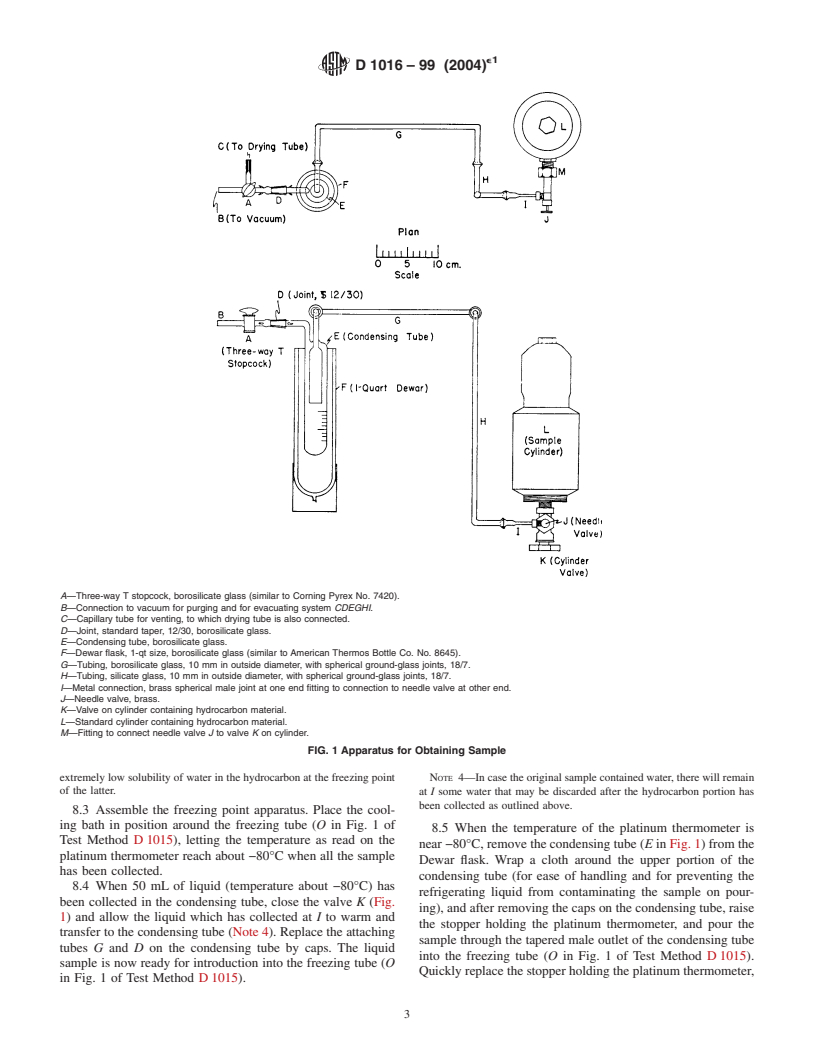
A—Three-way T stopcock, borosilicate glass (similar to Corning Pyrex No. 7420).
B—Connection to vacuum for purging and for evacuating system CDEGHI.
C—Capillary tube for venting, to which drying tube is also connected.
D—Joint, standard taper, 12/30, borosilicate glass.
E—Condensing tube, borosilicate glass.
F—Dewar flask, 1-qt size, borosilicate glass (similar to American Thermos Bottle Co. No. 8645).
G—Tubing, borosilicate glass, 10 mm in outside diameter, with spherical ground-glass joints, 18/7.
H—Tubing, silicate glass, 10 mm in outside diameter, with spherical ground-glass joints, 18/7.
I—Metal connection, brass spherical male joint at one end fitting to connection to needle valve at other end.
J—Needle valve, brass.
K—Valve on cylinder containing hydrocarbon material.
L—Standard cylinder containing hydrocarbon material.
M—Fitting to connect needle valve J to valve K on cylinder.
FIG. 1 Apparatus for Obtaining Sample
extremely low solubility of water in the hydrocarbon at the freezing point NOTE 4—Incasetheoriginalsamplecontainedwater,therewillremain
of the latter. at I some water that may be discarded after the hydrocarbon portion has
been collected as outlined above.
8.3 Assemble the freezing point apparatus. Place the cool-
ing bath in position around the freezing tube (O in Fig. 1 of
8.5 When the temperature of the platinum thermometer is
Test Method D1015), letting the temperature as read on the
near−80°C,removethecondensingtube(EinFig.1)fromthe
platinum thermometer reach about −80°C when all the sample
Dewar flask. Wrap a cloth around the upper portion of the
has been collected.
condensing tube (for ease of handling and for preventing the
8.4 When 50 mL of liquid (temperature about −80°C) has
refrigerating liquid from contaminating the sample on pour-
been collected in the condensing tube, close the valve K (Fig.
ing),andafterremovingthecapsonthecondensingtube,raise
1) and allow the liquid which has collected at I to warm and
the stopper holding the platinum thermometer, and pour the
transfer to the condensing tube (Note 4). Replace the attaching
sample through the tapered male outlet of the condensing tube
tubes G and D on the condensing tube by caps. The liquid
into the freezing tube (O in Fig. 1 of Test Method D1015).
sample is now ready for introduction into the freezing tube (O
Quicklyreplacethestopperholdingtheplatinumthermometer,
in Fig. 1 of Test Method D1015).
e1
D1016–99 (2004)
C—Dewar vessel, 1-qt capacity, borosilicate glass.
D—Clamp.
E—Distilling tube, borosilicate glass, 25 mm in outside diameter.
A—Standard-taper, ground-glass joint, 24/40, borosilicate glass
F—Standard-taper ground-glass joint, 24/40 borosilicate glass.
B—Distilling flask, round bottom, 200-mL capacity, borosilicate glass.
G—Tubing, 10 mm in outside diameter, borosilicate glass.
C—Tubing, 10 mm in outside diameter, borosilicate glass.
H, H8—Spherical ground-glass joints, 18/7, borosilicate glass.
D, D8—Spherical ground-glass joints, 18/7, borosilicate glass.
I—Tubing, 6 mm in outside diameter, borosilicate glass.
E—Dewar flask, 1-qt capacity, borosilicate glass.
J—Receiver, 35 mm in outside diameter, 150 mm in length, borosilicate glass.
F—Receiver, same as J in Fig. 2.
FIG. 2 Simple Distilling Apparatus for Normally Gaseous
FIG. 3 Simple Distilling Apparatus for Normally Liquid
Substances
Substances
9. Isobutane (Warning—Extremely flammable gas under
and start the stirrer, with dry air flowing into the upper portion
pressure. Vapor reduces oxygen available for breathing.)
ofthefreezingtubethroughM(Fig.1ofTestMethodD1015).
9.1 Determine the freezing point from freezing curves with
8.6 Because the material is normally gaseous at room
thecagestirrer,withacoolingbathofliquidnitrogen(orliquid
temperature, care should be taken in disposing of the sample
air), with a cooling rate of 0.3 to 0.8°C/min for the liquid near
safely.
the freezing point, and with crystallization induced immedi-
8.7 For n-butane, the freezing point for zero impurity, in air
ately below the freezing point by means of a cold rod.
at 1 atm, is as follows:
9.2 Obtain the samples as follows: Assemble the apparatus
t 5138.362 60.025°C (3)
f 0 for obtaining the sample as shown in Fig. 1, but with no
lubricant on the ground-glass joints and with the valve at the
and the cryoscopic constants are:
bottom of the cylinder, so that sampling is from the
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.