ASTM D1016-99
(Test Method)Standard Test Method for Purity of Hydrocarbons from Freezing Points
Standard Test Method for Purity of Hydrocarbons from Freezing Points
SCOPE
1.1 This test method covers the sampling and determination of purity of essentially pure compounds for which the freezing points for zero impurity and cryoscopic constants are given. The compounds to which the test method is applicable are: n-butane 1,3-butadiene isobutane isoprene(2-methyl-1,3-butadiene) n-pentane benzene isopentane toluene (methylbenzene) n-hexane ethylbenzene n-heptane o-xylene (1,2-dimethylbenzene) n-octane m-xylene (1,3-dimethylbenzene) 2,2,4-trimethylpentane p-xylene (1,4-dimethylbenzene) methylcyclohexane styrene (ethenylbenzene) isobutene
1.2 The values stated in SI units are to be regarded as the standard. The values in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see Sections 1, 6, 8, and 10 through 26. Note 2-This test method covers systems in which the impurities form with the major component a substantially ideal or sufficiently dilute solution, and also systems which deviate from the ideal laws, provided that, in the latter case, the lowering of the freezing point as a function of the concentration is known for each most probable impurity in the given substance.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation: D 1016 – 99
Standard Test Method for
Purity of Hydrocarbons from Freezing Points
This standard is issued under the fixed designation D 1016; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Summary of Test Method
1.1 This test method covers the sampling and determination 3.1 After measurement of the freezing point of the actual
of purity of essentially pure compounds for which the freezing sample, purity can be calculated from the value of the
points for zero impurity and cryoscopic constants are given. determined freezing point and the values given for the freezing
The compounds to which the test method is applicable are: point for zero impurity and for the applicable cryoscopic
(Warning: Extremely flammable liquids and liquefied gases.) constant or constants.
3.2 For the equilibrium between an infinitesimal amount of
n-butane 1,3-butadiene
isobutane isoprene(2-methyl-1,3-butadiene)
the crystalline phase of the major component and a liquid phase
n-pentane benzene
of the major component and one or more other components, the
isopentane toluene (methylbenzene)
thermodynamic relation between the temperature of equilib-
n-hexane ethylbenzene
n-heptane o-xylene (1,2-dimethylbenzene)
rium and the composition of the liquid phase is expressed by
n-octane m-xylene (1,3-dimethylbenzene)
the equation:
2,2,4-trimethylpentane p-xylene (1,4-dimethylbenzene)
methylcyclohexane styrene (ethenylbenzene)
21n N 521n ~1 2 N ! 5 A~t 2 t !@1 1 B~t 2 t ! 1 .# (1)
1 2 f 0 f f 0 f
isobutene
where:
1.2 The values stated in SI units are to be regarded as the
N 5 mole fraction of the major component,
standard. The values in parentheses are for information only.
N 5 (1 − N ) 5 sum of the mole fractions of all the other
2 1
1.3 This standard does not purport to address all of the
components,
safety concerns, if any, associated with its use. It is the
t 5 freezing point, in degrees Celsius, of the given
f
responsibility of the user of this standard to establish appro-
substance (in which the mole fraction of the major
priate safety and health practices and determine the applica-
component is N ), defined as the temperature at
bility of regulatory limitations prior to use. For specific hazard
which an infinitesimal amount of crystals of the
statements, see Sections 1, 6, 8, and 10-26.
major component is in thermodynamic equilibrium
NOTE 1—This test method covers systems in which the impurities form
with the liquid phase (see Note 3 of Test Method
with the major component a substantially ideal or sufficiently dilute
D 1015),
solution, and also systems which deviate from the ideal laws, provided
t 5 freezing point for zero impurity, in degrees Celsius,
f0
that, in the latter case, the lowering of the freezing point as a function of
for the major component when pure, that is, when N
the concentration is known for each most probable impurity in the given
1 51or N 5 0,
substance. 2
A 5 first or main cryoscopic constant, in mole fraction
2. Referenced Documents
per degree, and
B 5 secondary cryoscopic constant, in mole fraction per
2.1 ASTM Standards:
degree.
D 1015 Test Method for Freezing Points of High-Purity
3 Neglecting the higher terms not written in the brackets, Eq 1
Hydrocarbons
can be transformed to the equation:
log P 5 2.00000 2 ~A / 2.3026!~t 2 t !@1 1 B~t 2 t !# (2)
10 f 0 f f 0 f
This test method is under the jurisdiction of ASTM Committee D-2 on
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee
D02.04.0D on Physical Methods. For a more complete discussion of this test method, see Glasgow, Jr., A. R.,
Current edition approved Nov. 10, 1999. Published December 1999. Originally Streiff, A. J., and Rossini, F. D., “Determination of the Purity of Hydrocarbons by
published as D 1016 – 49 T. Last previous edition D 1016 – 94. Measurement of Freezing Points,” Journal of Research, JRNBA, National Institute
Numerical constants in this test method were taken from the most recently of Standards and Technology, Vol 35, No. 6, 1945, p. 355.
published data appearing in “Tables of Physical and Thermodynamic Properties of For details, see Taylor, W. J., and Rossini, F. D., “Theoretical Analysis of
Hydrocarbons and Related Compounds,” or ASTM DS 4A, Physical Constants of Time-Temperature Freezing and Melting Curves as Applied to Hydrocarbons,”
Hydrocarbons C to C , or both, prepared by the American Petroleum Institute, Journal of Research, JRNBA, Nat. Bureau Standards, Vol 32, No. 5, 1944, p. 197;
1 10
Research Project 44. also Lewis, G. N., and Randall, M., “Thermodynamics and the Free Energy of
Annual Book of ASTM Standards, Vol 05.01. Chemical Substances,” 1923, pp. 237, 238, McGraw-Hill Book Co., New York, NY.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 1016
5. Apparatus
where:
P 5 purity of the given substance in terms of mole percent
5.1 Sampling Apparatus, as shown in Fig. 1, for withdraw-
of the major component.
ing liquefied gases (for example, 1,3-butadiene) from pressure
storage cylinders.
4. Significance and Use
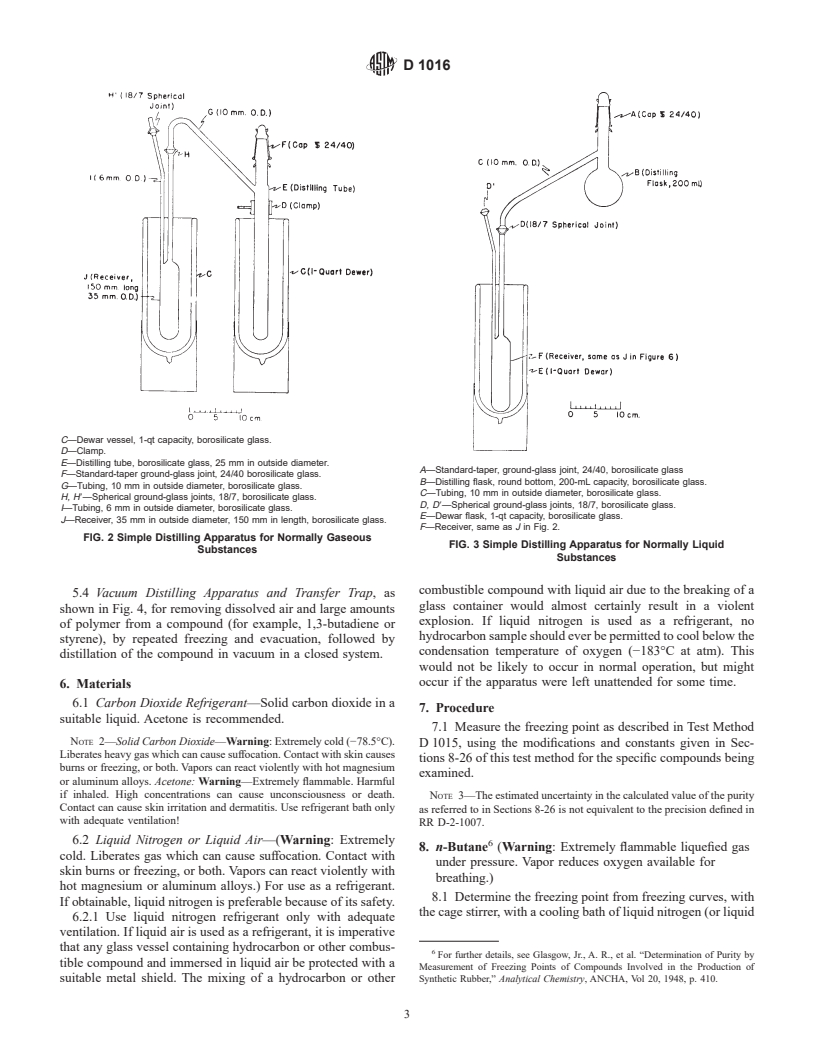
5.2 Distilling Apparatus, as shown in Fig. 2, for removing
4.1 The experimental procedures and physical constants
small amounts of polymer from low-boiling compounds (for
provided by this test method, when used in conjunction with
example, 1,3-butadiene) by simple distillation at atmospheric
Test Method D 1015, allow the determination of the purity of
pressure.
the material under test. A knowledge of the purity of these
5.3 Distilling Apparatus, as shown in Fig. 3, for removing
hydrocarbons is often needed to help control their manufacture
small amounts of polymer from compounds with boiling points
and to determine their suitability for use as reagent chemicals
or for conversion to other chemical intermediates or finished near room temperature (for example, isoprene) by distillation
products. at atmospheric pressure.
A—Three-way T stopcock, borosilicate glass (similar to Corning Pyrex No. 7420).
B—Connection to vacuum for purging and for evacuating system CDEGHI.
C—Capillary tube for venting, to which drying tube is also connected.
D—Joint, standard taper, 12/30, borosilicate glass.
E—Condensing tube, borosilicate glass.
F—Dewar flask, 1-qt size, borosilicate glass (similar to American Thermos Bottle Co. No. 8645).
G—Tubing, borosilicate glass, 10 mm in outside diameter, with spherical ground-glass joints, 18/7.
H—Tubing, silicate glass, 10 mm in outside diameter, with spherical ground-glass joints, 18/7.
I—Metal connection, brass spherical male joint at one end fitting to connection to needle valve at other end.
J—Needle valve, brass.
K—Valve on cylinder containing hydrocarbon material.
L—Standard cylinder containing hydrocarbon material.
M—Fitting to connect needle valve J to valve K on cylinder.
FIG. 1 Apparatus for Obtaining Sample
D 1016
C—Dewar vessel, 1-qt capacity, borosilicate glass.
D—Clamp.
E—Distilling tube, borosilicate glass, 25 mm in outside diameter.
A—Standard-taper, ground-glass joint, 24/40, borosilicate glass
F—Standard-taper ground-glass joint, 24/40 borosilicate glass.
B—Distilling flask, round bottom, 200-mL capacity, borosilicate glass.
G—Tubing, 10 mm in outside diameter, borosilicate glass.
C—Tubing, 10 mm in outside diameter, borosilicate glass.
H, H8—Spherical ground-glass joints, 18/7, borosilicate glass.
D, D8—Spherical ground-glass joints, 18/7, borosilicate glass.
I—Tubing, 6 mm in outside diameter, borosilicate glass.
E—Dewar flask, 1-qt capacity, borosilicate glass.
J—Receiver, 35 mm in outside diameter, 150 mm in length, borosilicate glass.
F—Receiver, same as J in Fig. 2.
FIG. 2 Simple Distilling Apparatus for Normally Gaseous
FIG. 3 Simple Distilling Apparatus for Normally Liquid
Substances
Substances
combustible compound with liquid air due to the breaking of a
5.4 Vacuum Distilling Apparatus and Transfer Trap,as
glass container would almost certainly result in a violent
shown in Fig. 4, for removing dissolved air and large amounts
explosion. If liquid nitrogen is used as a refrigerant, no
of polymer from a compound (for example, 1,3-butadiene or
hydrocarbon sample should ever be permitted to cool below the
styrene), by repeated freezing and evacuation, followed by
condensation temperature of oxygen (−183°C at atm). This
distillation of the compound in vacuum in a closed system.
would not be likely to occur in normal operation, but might
occur if the apparatus were left unattended for some time.
6. Materials
6.1 Carbon Dioxide Refrigerant—Solid carbon dioxide in a
7. Procedure
suitable liquid. Acetone is recommended.
7.1 Measure the freezing point as described in Test Method
NOTE 2—Solid Carbon Dioxide—Warning: Extremely cold (−78.5°C).
D 1015, using the modifications and constants given in Sec-
Liberates heavy gas which can cause suffocation. Contact with skin causes
tions 8-26 of this test method for the specific compounds being
burns or freezing, or both. Vapors can react violently with hot magnesium
examined.
or aluminum alloys. Acetone: Warning—Extremely flammable. Harmful
if inhaled. High concentrations can cause unconsciousness or death.
NOTE 3—The estimated uncertainty in the calculated value of the purity
Contact can cause skin irritation and dermatitis. Use refrigerant bath only
as referred to in Sections 8-26 is not equivalent to the precision defined in
with adequate ventilation!
RR D-2-1007.
6.2 Liquid Nitrogen or Liquid Air—(Warning: Extremely
8. n-Butane (Warning: Extremely flammable liquefied gas
cold. Liberates gas which can cause suffocation. Contact with
under pressure. Vapor reduces oxygen available for
skin burns or freezing, or both. Vapors can react violently with
breathing.)
hot magnesium or aluminum alloys.) For use as a refrigerant.
8.1 Determine the freezing point from freezing curves, with
If obtainable, liquid nitrogen is preferable because of its safety.
the cage stirrer, with a cooling bath of liquid nitrogen (or liquid
6.2.1 Use liquid nitrogen refrigerant only with adequate
ventilation. If liquid air is used as a refrigerant, it is imperative
that any glass vessel containing hydrocarbon or other combus-
For further details, see Glasgow, Jr., A. R., et al. “Determination of Purity by
tible compound and immersed in liquid air be protected with a
Measurement of Freezing Points of Compounds Involved in the Production of
suitable metal shield. The mixing of a hydrocarbon or other Synthetic Rubber,” Analytical Chemistry, ANCHA, Vol 20, 1948, p. 410.
D 1016
A, A8—Standard-taper ground-glass joints, 14/35 borosilicate glass. G and G8—Abestos pad.
B—Tubing, 27 mm in outside diameter, borosilicate glass. H, H8,H9—Stopcock, ground for high vacuum, borosilicate glass.
C, C8—Clamp. I—Spherical ground-glass joint, 18/7, borosilicate glass.
3 1
D— Brass cylinder, 273 mm (10 ⁄4 in.) in length, 28.6 mm (1 ⁄8 in.) in inside J—Condensing tube, used as trap (see E in Fig. 1).
diameter; for precautions in use of liquid nitrogen and liquid air, see R in K—Connection to vacuum system.
legend to Fig. 1 of Test Method D 1015 and Notes 2 and 3 of Test Method. L, L8—Stopcock, ground for high vacuum, borosilicate glass.
D 1015 M—Standard-taper ground-glass joint, 24/40 borosilicate glass.
D8— Brass cylinder, 254 mm (10 in.) in length, 47.6 mm (1 ⁄8 in.) in inside diameter, N—Receiver withdrawal, 36 mm in outside diameter, borosilicate glass.
(see D above). O—Dewar flask, 0.0005-m (1-pt) capacity, borosilicate glass.
E—Original sample. P—Connection to vacuum.
E8—Distilled sample. Q—Funnel with extension, 4 mm in inside diameter, borosilicate glass.
F, F8—Dewar flask, 0.0009-m (1-qt) capacity, borosilicate glass. R—Connection to drying tube, borosilicate glass.
FIG. 4 Apparatus for Simple Vacuum Distillation
air), with a cooling rate of 0.3 to 0.8°C/min for the liquid near 8.4 When 50 mL of liquid (temperature about − 80°C) has
the freezing point, and with crystallization induced immedi- been collected in the condensing tube, close the valve K (Fig.
ately below the freezing point by means of a cold rod.
1) and allow the liquid which has collected at I to warm and
8.2 The method of obtaining the samples shall be as transfer to the condensing tube (Note 5). Replace the attaching
follows: Assemble the apparatus for obtaining the sample as
tubes G and D on the condensing tube by caps. The liquid
shown in Fig. 1, but with no lubricant on the ground-glass
sample is now ready for introduction into the freezing tube (O
joints and with the valve at the bottom of the cylinder, so that
in Fig. 1 of Test Method D 1015).
sampling is from the liquid phase. Attach to C an absorption
NOTE 5—In case the original sample contained water, there will remain
tube containing anhydrous calcium sulfate or other suitable
at I some water that may be discarded after the hydrocarbon portion has
desiccant (except magnesium perchlorate) so that water is not
been collected as outlined above.
introduced into the system (Note 4). Fill the flask F with the
carbon dioxide refrigerant to within about 51 mm (2 in.) of the 8.5 When the temperature of the platinum thermometer is
top. After about 20 or 30 min, when the system will have near − 80°C, remove the condensing tube (E in Fig. 1) from the
cooled sufficiently, remove the absorption tube and begin the
Dewar flask. Wrap a cloth around the upper portion of the
collection of liquid n-butane by opening the valve K and condensing tube (for ease of handling and for preventing the
adjusting the needle valve J so that the sample is collected at
refrigerating liquid from contaminating the sample on pour-
a rate of 1 to 2 mL (liquid)/min in the condensing tube E.
ing), and after removing the caps on the condensing tube, raise
the stopper holding the platinum thermometer, and pour the
NOTE 4—However, if some water does condense with the hydrocarbon,
sample through the tapered male outlet of the condensing tube
the freezing point will not be affected significantly because of the
extremely low solubility of water in the hydrocarbon at the freezing point into the freezing tube ( O in Fig. 1 of Test Method D 1015).
of the latter.
Quickly replace the stopper holding the platinum thermometer
and start the stirrer, with dry air flowing into the upper portion
8.3 Assemble the freezing point apparatus. Place the cool-
of the freezing tube thr
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.