ASTM F2791-09
(Guide)Standard Guide for Assessment of Surface Texture of Non-Porous Biomaterials in Two Dimensions
Standard Guide for Assessment of Surface Texture of Non-Porous Biomaterials in Two Dimensions
SIGNIFICANCE AND USE
The term “surface texture” is used to describe the local deviations of a surface from an ideal shape. Surface texture usually consists of long wavelength repetitive features that occur as results of chatter, vibration, or heat treatments during the manufacture of implants. Short wavelength features superimposed on the long wavelength features of the surface, which arise from polishing or etching of the implant, are referred to as roughness.
This guide provides an overview of techniques that are available for measuring the surface in terms of Cartesian coordinates and the parameters used to describe surface texture. It is important to appreciate that it is not possible to measure surface texture per se, but to derive values for parameters that can be used to describe it.
SCOPE
1.1 This guide describes some of the more common methods that are available for measuring the topographical features of a surface and provides an overview of the parameters that are used to quantify them. Being able to reliably derive a set of parameters that describe the texture of biomaterial surfaces is a key aspect in the manufacture of safe and effective implantable medical devices that have the potential to trigger an adverse biological reaction in situ.
1.2 This guide is not intended to apply to porous structures with average pore dimensions in excess of approximately 50 nm (0.05 μm).
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F2791 −09
StandardGuide for
Assessment of Surface Texture of Non-Porous Biomaterials
in Two Dimensions
This standard is issued under the fixed designation F2791; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2.2 Other Standards:
ISO 3274 Geometrical Product Specifications (GPS)—
1.1 This guide describes some of the more common meth-
Surface Texture: Profile Method—Nominal Characteris-
ods that are available for measuring the topographical features
tics of Contact (Stylus) Instruments
of a surface and provides an overview of the parameters that
ISO 4287 Geometrical Product Specifications (GPS)—
are used to quantify them. Being able to reliably derive a set of
Surface Texture: Profile Method—Terms, Definitions and
parameters that describe the texture of biomaterial surfaces is
Surface Texture Parameters
a key aspect in the manufacture of safe and effective implant-
ISO 4288 Geometrical Product Specifications (GPS)—
able medical devices that have the potential to trigger an
Surface Texture: Profile Method—Rules and Procedures
adverse biological reaction in situ.
for the Assessment of Surface Texture
1.2 This guide is not intended to apply to porous structures
ISO 13565–1 Geometrical Product Specifications (GPS)—
with average pore dimensions in excess of approximately 50
SurfaceTexture: Profile Method—Surfaces Having Strati-
nm (0.05 µm).
fiedFunctionalProperties;FilteringandGeneralMeasure-
ment Conditions
1.3 The values stated in SI units are to be regarded as
standard. No other units of measurement are included in this
3. Terminology
standard.
3.1 Definitions of Terms Specific to This Standard:
1.4 This standard does not purport to address all of the
3.1.1 biocompatible, adj—a material may be considered
safety concerns, if any, associated with its use. It is the
biocompatibleifthematerialsperformwithanappropriatehost
responsibility of the user of this standard to establish appro-
response in a specific application. F2312
priate safety and health practices and determine the applica-
3.1.2 biomaterial, n—any substance (other than a drug),
bility of regulatory limitations prior to use.
synthetic or natural, that can be used as a system or part of a
system that treats, augments, or replaces any tissue, organ, or
2. Referenced Documents
function of the body. F2664
2.1 ASTM Standards:
3.1.3 evaluation length, ln, n—length in the direction of the
C813 Test Method for Hydrophobic Contamination on Glass
x-axis used to assess the profile under evaluation.
by Contact Angle Measurement
3.1.3.1 Discussion—The evaluation length may contain one
F2312 Terminology Relating to Tissue Engineered Medical
or more sampling lengths. ISO 4287
Products
F2450 Guide for Assessing Microstructure of Polymeric
3.1.4 hydrophilic, adj—having a strong affinity for water;
Scaffolds for Use in Tissue-Engineered Medical Products
wettable.
F2664 Guide for Assessing the Attachment of Cells to
3.1.4.1 Discussion—Hydrophilic surfaces exhibit zero con-
Biomaterial Surfaces by Physical Methods
tact angles. C813
3.1.5 hydrophobic, adj—having little affinity for water;
nonwettable.
This guide is under the jurisdiction of ASTM Committee F04 on Medical and
3.1.5.1 Discussion—Hydrophobic surfaces exhibit contact
Surgical Materials and Devices and is the direct responsibility of Subcommittee
anglesappreciablygreaterthanzero:generallygreaterthan45°
F04.42 on Biomaterials and Biomolecules for TEMPs.
for the advancing angle. C813
Current edition approved Aug. 1, 2009. Published September 2009. DOI:
10.1520/F2791-09.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F2791−09
3.1.6 implant, n—a substance or object that is put in the 4.2 This guide provides an overview of techniques that are
body as a prosthesis, or for treatment or diagnosis. F2664 available for measuring the surface in terms of Cartesian
coordinates and the parameters used to describe surface tex-
3.1.7 lay, n—the direction of the predominant surface
ture. It is important to appreciate that it is not possible to
pattern. ISO 13565–1
measure surface texture per se, but to derive values for
3.1.8 primary profile, n—the profile after application of the
parameters that can be used to describe it.
short wavelength filters. ISO 3274
3.1.9 profile peak, n—an outwardly directed (from the
5. The Relationship Between Surface Texture, Surface
material to the surrounding medium) portion of the assessed Chemistry, Surface Energy, and Biocompatibility
profileconnectingtwoadjacentpointsoftheintersectionofthe
5.1 The biocompatibility of materials is influenced by many
profile with the x-axis. ISO 4287
factors such as size, shape, material bulk, and surface chemical
3.1.10 profile valley, n—an inwardly directed (from sur-
composition, surface energy, and surface topography. Chang-
rounding medium to material) portion of the assessed profile
ing any one of these related characteristics of a biocompatible
connecting two adjacent points of the intersection of the
material can have a significant effect on cell behavior. The
assessed profile with the x-axis. ISO 4287
response of a cell to a biomaterial can be assessed by
measuring the adhesive strength between it and the underlying
3.1.11 real surface, n—surface limiting the body and sepa-
surface, monitoring changes in its shape or in the expression of
rating it from the surrounding medium. ISO 4287
biomarkers.
3.1.12 sampling length, lr, n—length in the direction of the
x-axis used for identifying the irregularities characterizing the 5.2 The chemical species present on a surface can be
mapped in detail using surface sensitive analysis techniques
profile under evaluation. ISO 4287
(for example, X-ray photoelectron spectroscopy where the
3.1.13 scaffold, n—a support, delivery vehicle or metric for
penetration depth is 10 nm or below (1)). The chemical
facilitating the migration, binding, or transport of cells or
species present on the surface together with the surface
bioactive molecules used to replace, repair, or regenerate
topography determine how hydrophilic the surface is. Measur-
tissues. F2450
ing the contact angle between the surface and a fluid, usually
3.1.14 surface profile, n—profile that results from the inter-
water,canassessthedegreeofhydrophilicityofasurface.Care
section of the real surface by a specified plane.
should be taken when comparing contact angle measurements
3.1.14.1 Discussion—In practice, it is usual to choose a
made on different surfaces, as the relative contributions from
plane with a normal that nominally lies parallel to the real
the surface chemistry and texture are unlikely to be the same.
surface and in a suitable direction. ISO 4287
6. Surfaces and Surface Profiles
4. Significance and Use
6.1 Conventionally surfaces are described in Cartesian co-
4.1 The term “surface texture” is used to describe the local
ordinates where the x-axis is defined as being perpendicular to
deviations of a surface from an ideal shape. Surface texture
the lay direction. The y-axis is in plane and is perpendicular to
usually consists of long wavelength repetitive features that
the x-axis direction. The z-axis is out of plane. The profile of a
occur as results of chatter, vibration, or heat treatments during
surface that has a uniform, non-directional texture can be
the manufacture of implants. Short wavelength features super-
imposed on the long wavelength features of the surface, which
arisefrompolishingoretchingoftheimplant,arereferredtoas 4
The boldface numbers in parentheses refer to a list of references at the end of
roughness. this standard.
NOTE 1—The surface shown in (A) has no directionality or lay, therefore profiles can be oriented at any angle. Profiles (dashed line arrow) are drawn
perpendicular to the lay (solid line arrow) in surfaces that have directionality (B).
FIG. 1Profile Orientation and Surface Features
F2791−09
measured at any in plane orientation (see Fig. 1(A)); however, faithfully reproduced due to limitations of the measurement
several profiles at different orientations should be measured to method, for example, an inability to track the sides of steep
find the maximum amplitude (see Fig. 1(A)). For patterned valleys that is in essence a form of filtering. This topic is
surfacesthathaveperiodicfeatures,alay,theorientationofthe further discussed in Section 11.
profile is at right angles to it (see Fig. 1(B)).
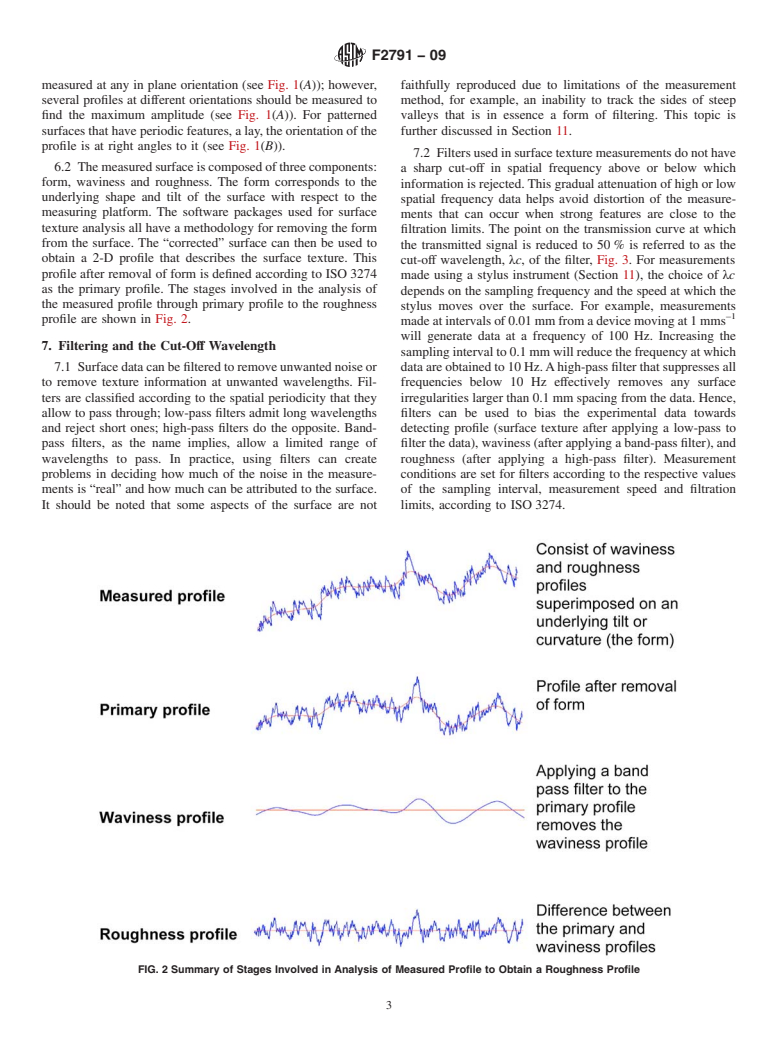
7.2 Filtersusedinsurfacetexturemeasurementsdonothave
6.2 Themeasuredsurfaceiscomposedofthreecomponents:
a sharp cut-off in spatial frequency above or below which
form, waviness and roughness. The form corresponds to the
information is rejected.This gradual attenuation of high or low
underlying shape and tilt of the surface with respect to the
spatial frequency data helps avoid distortion of the measure-
measuring platform. The software packages used for surface
ments that can occur when strong features are close to the
texture analysis all have a methodology for removing the form
filtration limits. The point on the transmission curve at which
from the surface. The “corrected” surface can then be used to
the transmitted signal is reduced to 50 % is referred to as the
obtain a 2-D profile that describes the surface texture. This
cut-off wavelength, λc, of the filter, Fig. 3. For measurements
profile after removal of form is defined according to ISO 3274
made using a stylus instrument (Section 11), the choice of λc
as the primary profile. The stages involved in the analysis of
depends on the sampling frequency and the speed at which the
the measured profile through primary profile to the roughness
stylus moves over the surface. For example, measurements
–1
profile are shown in Fig. 2.
madeatintervalsof0.01mmfromadevicemovingat1mms
will generate data at a frequency of 100 Hz. Increasing the
7. Filtering and the Cut-Off Wavelength
samplingintervalto0.1mmwillreducethefrequencyatwhich
7.1 Surfacedatacanbefilteredtoremoveunwantednoiseor dataareobtainedto10Hz.Ahigh-passfilterthatsuppressesall
to remove texture information at unwanted wavelengths. Fil- frequencies below 10 Hz effectively removes any surface
ters are classified according to the spatial periodicity that they irregularities larger than 0.1 mm spacing from the data. Hence,
allow to pass through; low-pass filters admit long wavelengths filters can be used to bias the experimental data towards
and reject short ones; high-pass filters do the opposite. Band- detecting profile (surface texture after applying a low-pass to
pass filters, as the name implies, allow a limited range of filterthedata),waviness(afterapplyingaband-passfilter),and
wavelengths to pass. In practice, using filters can create roughness (after applying a high-pass filter). Measurement
problems in deciding how much of the noise in the measure- conditions are set for filters according to the respective values
ments is “real” and how much can be attributed to the surface. of the sampling interval, measurement speed and filtration
It should be noted that some aspects of the surface are not limits, according to ISO 3274.
FIG. 2Summary of Stages Involved in Analysis of Measured Profile to Obtain a Roughness Profile
F2791−09
FIG. 350% Reduction in Transmission Curve
7.3 ISO 4287 specifies that 2-D roughness parameters need 8.1.2 Spatial parameters, which describe in-plane variations
to be determined over five sequential sampling lengths, lr, of surface texture; and
unless otherwise specified. This grouping of five serial sam- 8.1.3 Hybrid parameters, which combine both amplitude
pling lengths is referred to as the evaluation length, ln. The and spatial information, for example, mean slope.
sampling length varies according to the length scale of the
8.2 Ra—The most widely used parameter to quantify sur-
texture being assessed; larger features require a long sampling
face texture is the arithmetical mean deviation of the absolute
length.Guidanceastowhichsamplinglengthtouseforagiven
ordinate values, Z(x), of the profile from a center line (see
range of feature sizes is shown in Table 1. It may be necessary
Table 2 and Fig. 5). Despite its common usage, Ra does not
to perform one or more iterations to identify the best value for
provideatrulyaccuraterepresentationofasurfaceprofilesince
lr. This can be achieved by calculating the mean width of a
any information regarding peak heights or valley depths can be
profile element, RSm (see Fig. 4), from a measured profile
lost in its derivation. This insensitivity to surface texture is
where the value for lr is based on a best guess. This initial
apparent in Fig. 6, which shows that quite different profiles can
iteration will enable a new value for RSm to be determined and
have the same Ra value. The statistical significance of Ra is
that leads to a potential revision of lr according to Table 1.
improved by averaging the values obtained for each of the five
sampling lengths.
8. Quantification of Surface Profiles
8.3 Rq—The root-mean-square value of all distances of the
8.1 Parameters that are used to characterize 2-D surface
measured profile away from the center line, Rq, although
profiles are grouped as:
similar in terms of its derivation to Ra has a subtle but
8.1.1 Amplitude parameters, which are measures of varia-
significant difference. The deviations of the peak heights and
tions in profile height. These parameters are split into two
valley depths from the midline appear as a squared term in Rq.
subclasses: averaging parameters, and peak and valley param-
Thatincreasesitssensitivitytohighpeaksordeepvalleys.This
eters;
sensitivity can be useful, but it should be noted that the
presenceofaforeignbody,forexample,hairorascratchinthe
TABLE 1 Guide to Choosing Sampling Lengths for the surface can have a significant influence on the value of Rq.
Measurement of Periodic Profiles
8.4 Rsk—Skewness, the distribution of peak heights and
NOTE 1—Based on ISO 4288. The evaluation length is usually taken to
valley depths provides valuable information about surface
be five times the sampling length.
texture. A surface th
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.