ASTM F2091-15
(Specification)Standard Specification for Acetabular Prostheses (Withdrawn 2023)
Standard Specification for Acetabular Prostheses (Withdrawn 2023)
ABSTRACT
This specification covers acetabular resurfacing devices used to provide a functioning articulation between the bones of the acetabulum and the femur. Acetabular prostheses included within the scope of this specification are intended for mechanical fixation between the prosthesis and host bone, by the use of bone cement or through biological fixation. Acetabular prostheses shall be classified as: Type I and Type II. The following test methods shall be performed: mechanical strength; corrosion resistance; biocompatibility; structural requirements; metal and ceramic coating or surface texture integrity; component disassociation; fixation failure; device fracture; and articular surface wear.
SCOPE
1.1 This specification covers acetabular resurfacing devices used to provide a functioning articulation between the bones of the acetabulum and the femur.
1.2 This specification is intended to provide basic descriptions of materials and device geometry. Additionally, those characteristics determined to be important to in vivo performance of the device are defined.
1.3 Acetabular prostheses included within the scope of this specification are intended for fixation by press-fit between the prosthesis and host bone, the use of bone cement, the use of bone screws or similar means of mechanical fixation, or through biological fixation of host bone and/or soft connective tissue into a porous surface.
1.4 Custom (designed explicitly for a single patient), revision, or constrained acetabular prostheses are not covered within the scope of this specification.
1.5 This specification does not cover the details for quality assurance, design control, production control contained in 21 CFR 820 (Quality System Regulation) and ISO 9001.
WITHDRAWN RATIONALE
This specification covered acetabular resurfacing devices used to provide a functioning articulation between the bones of the acetabulum and the femur.
Formerly under the jurisdiction of Committee F04 on Medical and Surgical Materials and Devices, this specification was withdrawn in December 2023. This standard is being withdrawn without replacement due to its limited use by industry.
General Information
Relations
Buy Standard
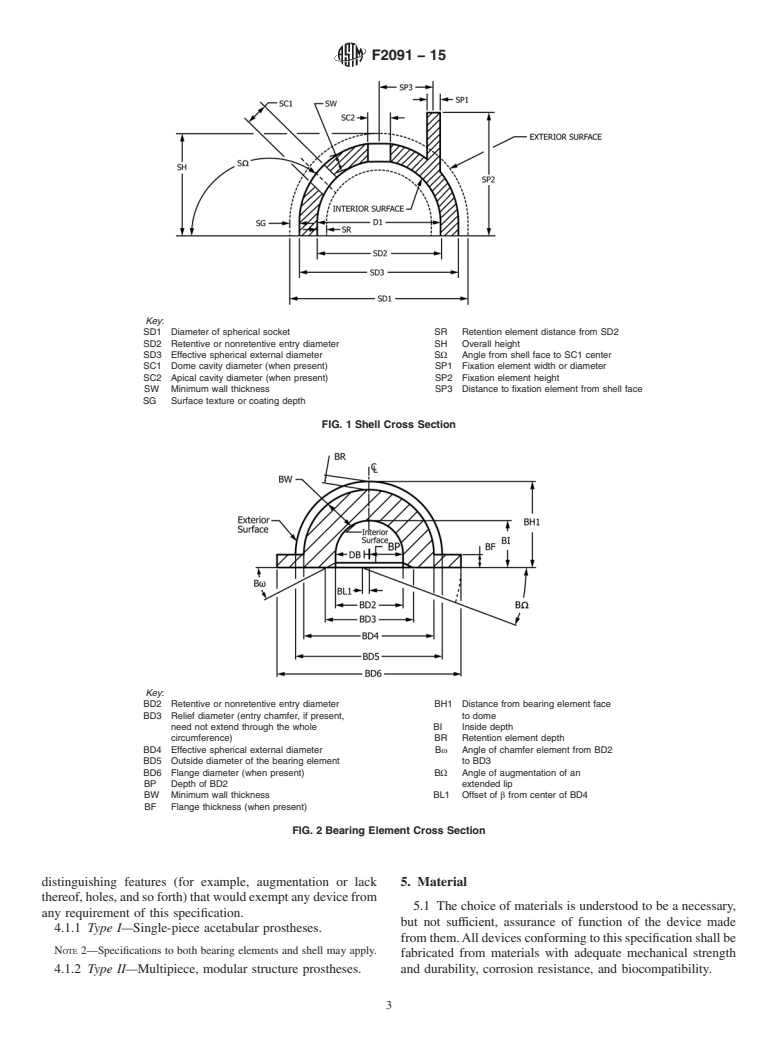
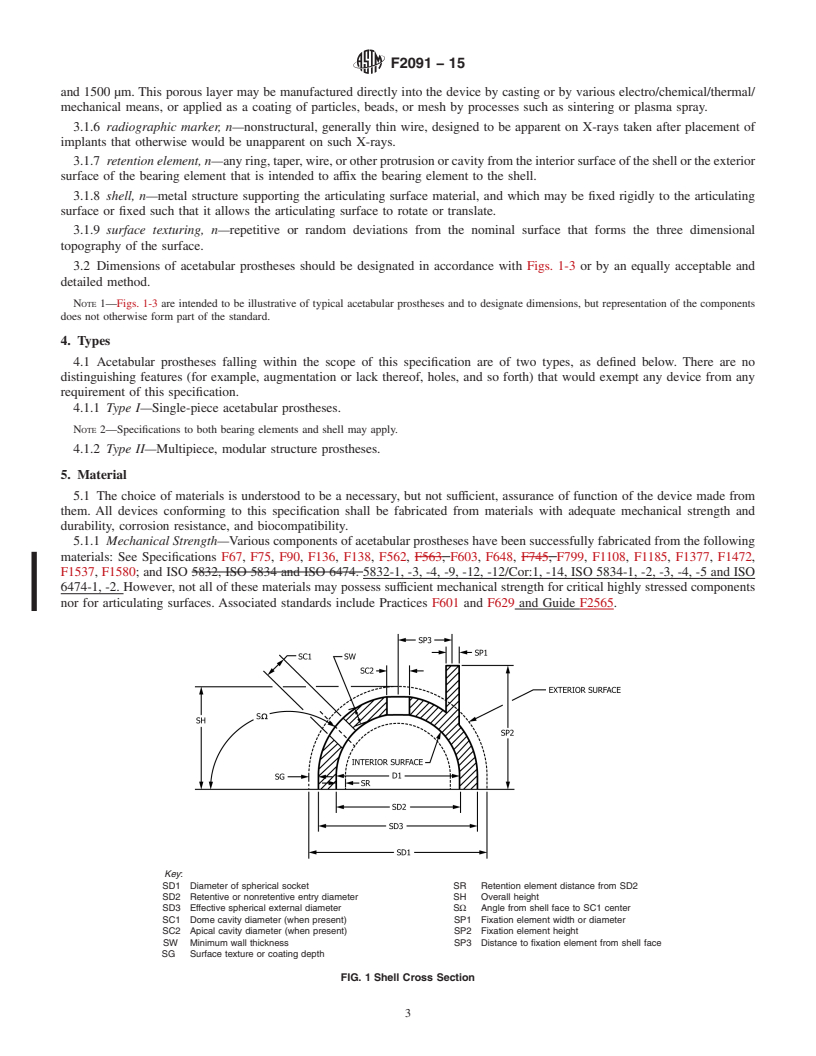
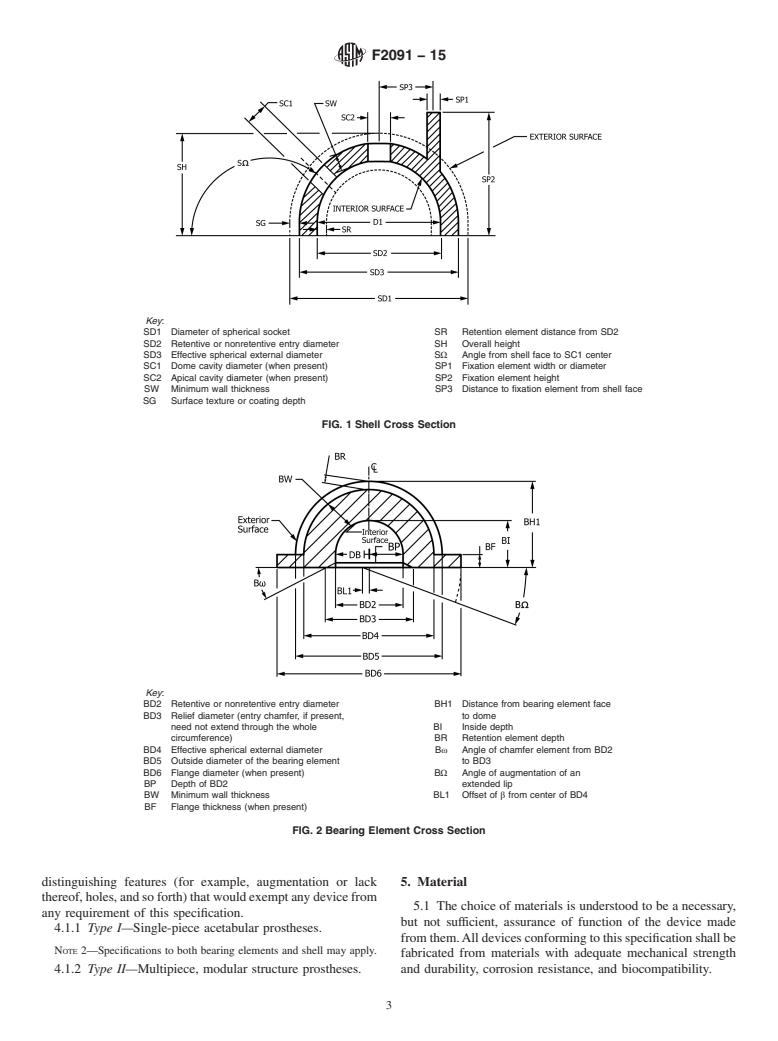
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation:F2091 −15
Standard Specification for
1
Acetabular Prostheses
This standard is issued under the fixed designation F2091; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope F90 Specification for Wrought Cobalt-20Chromium-
15Tungsten-10NickelAlloy for Surgical ImplantApplica-
1.1 This specification covers acetabular resurfacing devices
tions (UNS R30605)
used to provide a functioning articulation between the bones of
F136 Specification for Wrought Titanium-6Aluminum-
the acetabulum and the femur.
4Vanadium ELI (Extra Low Interstitial)Alloy for Surgical
1.2 This specification is intended to provide basic descrip-
Implant Applications (UNS R56401)
tions of materials and device geometry. Additionally, those
F138 Specification for Wrought 18Chromium-14Nickel-
characteristics determined to be important to in vivo perfor-
2.5Molybdenum Stainless Steel Bar andWire for Surgical
mance of the device are defined.
Implants (UNS S31673)
1.3 Acetabular prostheses included within the scope of this F562 Specification for Wrought 35Cobalt-35Nickel-
specification are intended for fixation by press-fit between the
20Chromium-10Molybdenum Alloy for Surgical Implant
prosthesis and host bone, the use of bone cement, the use of Applications (UNS R30035)
bone screws or similar means of mechanical fixation, or
F601 Practice for Fluorescent Penetrant Inspection of Me-
through biological fixation of host bone and/or soft connective tallic Surgical Implants
tissue into a porous surface.
F603 Specification for High-Purity Dense Aluminum Oxide
for Medical Application
1.4 Custom (designed explicitly for a single patient),
F629 Practice for Radiography of Cast Metallic Surgical
revision, or constrained acetabular prostheses are not covered
Implants
within the scope of this specification.
F648 Specification for Ultra-High-Molecular-Weight Poly-
1.5 This specification does not cover the details for quality
ethylene Powder and Fabricated Form for Surgical Im-
assurance, design control, production control contained in 21
plants
CFR 820 (Quality System Regulation) and ISO 9001.
F746 Test Method for Pitting or Crevice Corrosion of
Metallic Surgical Implant Materials
2. Referenced Documents
F748 PracticeforSelectingGenericBiologicalTestMethods
2
2.1 ASTM Standards:
for Materials and Devices
F67 Specification for Unalloyed Titanium, for Surgical Im-
F799 Specification for Cobalt-28Chromium-6Molybdenum
plant Applications (UNS R50250, UNS R50400, UNS
Alloy Forgings for Surgical Implants (UNS R31537,
R50550, UNS R50700)
R31538, R31539)
F75 Specification for Cobalt-28 Chromium-6 Molybdenum
F981 Practice for Assessment of Compatibility of Biomate-
Alloy Castings and Casting Alloy for Surgical Implants
rials for Surgical Implants with Respect to Effect of
(UNS R30075)
Materials on Muscle and Bone
F86 Practice for Surface Preparation and Marking of Metal-
F983 Practice for Permanent Marking of Orthopaedic Im-
lic Surgical Implants
plant Components
F1044 Test Method for Shear Testing of Calcium Phosphate
1
This specification is under the jurisdiction of ASTM Committee F04 on Coatings and Metallic Coatings
Medical and Surgical Materials and Devices and is the direct responsibility of
F1108 Specification for Titanium-6Aluminum-4Vanadium
Subcommittee F04.22 on Arthroplasty.
Alloy Castings for Surgical Implants (UNS R56406)
Current edition approved March 15, 2015. Published May 2015. Originally
F1147 Test Method for Tension Testing of Calcium Phos-
approved in 2001. Last previous edition approved in 2012 as F2091 – 01 (2012).
DOI: 10.1520/F2091-15.
phate and Metallic Coatings
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
F1160 Test Method for Shear and Bending Fatigue Testing
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
of Calcium Phosphate and Metallic Medical and Compos-
Standards volume information, refer to the standard’s Document Summary page on
ite Calcium Phosphate/Metallic Coatings
the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2091−15
F1185 Specification for Composition of Hydroxylapatite for 2.3 Code of Federal Regulations:
4
Surgical Implants 21 CFR 820 Quality System Regulation
F1377 Specification for Cobalt-28Chromium-6Molybdenum
3. Terminology
Powder for Coating of Orthopedic Implants (UN
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F2091 − 01 (Reapproved 2012) F2091 − 15
Standard Specification for
1
Acetabular Prostheses
This standard is issued under the fixed designation F2091; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This specification covers acetabular resurfacing devices used to provide a functioning articulation between the bones of the
acetabulum and the femur.
1.2 This specification is intended to provide basic descriptions of materials and device geometry. Additionally, those
characteristics determined to be important to in vivo performance of the device are defined.
1.3 Acetabular prostheses included within the scope of this specification are intended for mechanical fixation by press-fit
between the prosthesis and host bone, by the use of bone cement or through biological fixation.cement, the use of bone screws or
similar means of mechanical fixation, or through biological fixation of host bone and/or soft connective tissue into a porous surface.
1.4 Custom (designed explicitly for a single patient), revision, or constrained acetabular prostheses are not covered within the
scope of this specification.
1.5 This specification does not cover the details for quality assurance, design control, production control contained in 21 CFR
820 (Quality System Regulation) and ISO 9001.
2. Referenced Documents
2
2.1 ASTM Standards:
F67 Specification for Unalloyed Titanium, for Surgical Implant Applications (UNS R50250, UNS R50400, UNS R50550, UNS
R50700)
F75 Specification for Cobalt-28 Chromium-6 Molybdenum Alloy Castings and Casting Alloy for Surgical Implants (UNS
R30075)
F86 Practice for Surface Preparation and Marking of Metallic Surgical Implants
F90 Specification for Wrought Cobalt-20Chromium-15Tungsten-10Nickel Alloy for Surgical Implant Applications (UNS
R30605)
F136 Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant
Applications (UNS R56401)
F138 Specification for Wrought 18Chromium-14Nickel-2.5Molybdenum Stainless Steel Bar and Wire for Surgical Implants
(UNS S31673)
F562 Specification for Wrought 35Cobalt-35Nickel-20Chromium-10Molybdenum Alloy for Surgical Implant Applications
(UNS R30035)
F563 Specification for Wrought Cobalt-20Nickel-20Chromium-3.5Molybdenum-3.5Tungsten-5Iron Alloy for Surgical Implant
3
Applications (UNS R30563) (Withdrawn 2005)
F601 Practice for Fluorescent Penetrant Inspection of Metallic Surgical Implants
F603 Specification for High-Purity Dense Aluminum Oxide for Medical Application
F629 Practice for Radiography of Cast Metallic Surgical Implants
F648 Specification for Ultra-High-Molecular-Weight Polyethylene Powder and Fabricated Form for Surgical Implants
F745 Specification for 18Chromium-12.5Nickel-2.5Molybdenum Stainless Steel for Cast and Solution-Annealed Surgical
3
Implant Applications (Withdrawn 2012)
F746 Test Method for Pitting or Crevice Corrosion of Metallic Surgical Implant Materials
1
This specification is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.22 on Arthroplasty.
Current edition approved Jan. 15, 2012March 15, 2015. Published January 2012May 2015. Originally approved in 2001. Last previous edition approved in 20062012 as
F2091 – 01 (2006).(2012). DOI: 10.1520/F2091-01R11.10.1520/F2091-15.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’sstandard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2091 − 15
F748 Practice for Selecting Generic Biological Test Methods for Materials and Devices
F799 Specification for Cobalt-28Chromium-6Molybdenum Alloy Forgings for Surgical Implants (UNS R31537, R31538,
R31539)
F981 Practice for Assessment of Compatibility of Biomaterials for Surgical Implants with Respect to Effect of Materials on
Muscle and Bone
F983 Practice for Permanent Marking of Orthopaedic Implant Components
F1044 Test Method for Shear Testing of Calcium Phosphate Coatings and Metallic Coatings
F1108 Specification for
...
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2091 − 15
Standard Specification for
1
Acetabular Prostheses
This standard is issued under the fixed designation F2091; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope F90 Specification for Wrought Cobalt-20Chromium-
15Tungsten-10Nickel Alloy for Surgical Implant Applica-
1.1 This specification covers acetabular resurfacing devices
tions (UNS R30605)
used to provide a functioning articulation between the bones of
F136 Specification for Wrought Titanium-6Aluminum-
the acetabulum and the femur.
4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical
1.2 This specification is intended to provide basic descrip-
Implant Applications (UNS R56401)
tions of materials and device geometry. Additionally, those
F138 Specification for Wrought 18Chromium-14Nickel-
characteristics determined to be important to in vivo perfor-
2.5Molybdenum Stainless Steel Bar and Wire for Surgical
mance of the device are defined.
Implants (UNS S31673)
1.3 Acetabular prostheses included within the scope of this
F562 Specification for Wrought 35Cobalt-35Nickel-
specification are intended for fixation by press-fit between the 20Chromium-10Molybdenum Alloy for Surgical Implant
prosthesis and host bone, the use of bone cement, the use of
Applications (UNS R30035)
bone screws or similar means of mechanical fixation, or F601 Practice for Fluorescent Penetrant Inspection of Me-
through biological fixation of host bone and/or soft connective
tallic Surgical Implants
tissue into a porous surface.
F603 Specification for High-Purity Dense Aluminum Oxide
for Medical Application
1.4 Custom (designed explicitly for a single patient),
F629 Practice for Radiography of Cast Metallic Surgical
revision, or constrained acetabular prostheses are not covered
Implants
within the scope of this specification.
F648 Specification for Ultra-High-Molecular-Weight Poly-
1.5 This specification does not cover the details for quality
ethylene Powder and Fabricated Form for Surgical Im-
assurance, design control, production control contained in 21
plants
CFR 820 (Quality System Regulation) and ISO 9001.
F746 Test Method for Pitting or Crevice Corrosion of
Metallic Surgical Implant Materials
2. Referenced Documents
F748 Practice for Selecting Generic Biological Test Methods
2
2.1 ASTM Standards:
for Materials and Devices
F67 Specification for Unalloyed Titanium, for Surgical Im-
F799 Specification for Cobalt-28Chromium-6Molybdenum
plant Applications (UNS R50250, UNS R50400, UNS
Alloy Forgings for Surgical Implants (UNS R31537,
R50550, UNS R50700)
R31538, R31539)
F75 Specification for Cobalt-28 Chromium-6 Molybdenum
F981 Practice for Assessment of Compatibility of Biomate-
Alloy Castings and Casting Alloy for Surgical Implants
rials for Surgical Implants with Respect to Effect of
(UNS R30075)
Materials on Muscle and Bone
F86 Practice for Surface Preparation and Marking of Metal-
F983 Practice for Permanent Marking of Orthopaedic Im-
lic Surgical Implants
plant Components
F1044 Test Method for Shear Testing of Calcium Phosphate
1
This specification is under the jurisdiction of ASTM Committee F04 on
Coatings and Metallic Coatings
Medical and Surgical Materials and Devices and is the direct responsibility of
F1108 Specification for Titanium-6Aluminum-4Vanadium
Subcommittee F04.22 on Arthroplasty.
Alloy Castings for Surgical Implants (UNS R56406)
Current edition approved March 15, 2015. Published May 2015. Originally
F1147 Test Method for Tension Testing of Calcium Phos-
approved in 2001. Last previous edition approved in 2012 as F2091 – 01 (2012).
DOI: 10.1520/F2091-15.
phate and Metallic Coatings
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
F1160 Test Method for Shear and Bending Fatigue Testing
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
of Calcium Phosphate and Metallic Medical and Compos-
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. ite Calcium Phosphate/Metallic Coatings
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2091 − 15
F1185 Specification for Composition of Hydroxylapatite for 2.3 Code of Federal Regulations:
4
Surgical Implants 21 CFR 820 Quality System Regulation
F1377 Specification for Cobalt-28Chromium-6Molybdenum
3. Terminology
Powder for Coating of Orthopedic Implants (UNS
3.1 Definitions:
R30075)
3.1.1 bearing element, n—articulating surface element be-
F1472 Specification for Wrought T
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.