ASTM F2091-01(2012)
(Specification)Standard Specification for Acetabular Prostheses
Standard Specification for Acetabular Prostheses
ABSTRACT
This specification covers acetabular resurfacing devices used to provide a functioning articulation between the bones of the acetabulum and the femur. Acetabular prostheses included within the scope of this specification are intended for mechanical fixation between the prosthesis and host bone, by the use of bone cement or through biological fixation. Acetabular prostheses shall be classified as: Type I and Type II. The following test methods shall be performed: mechanical strength; corrosion resistance; biocompatibility; structural requirements; metal and ceramic coating or surface texture integrity; component disassociation; fixation failure; device fracture; and articular surface wear.
SCOPE
1.1 This specification covers acetabular resurfacing devices used to provide a functioning articulation between the bones of the acetabulum and the femur.
1.2 This specification is intended to provide basic descriptions of materials and device geometry. Additionally, those characteristics determined to be important to in vivo performance of the device are defined.
1.3 Acetabular prostheses included within the scope of this specification are intended for mechanical fixation between the prosthesis and host bone, by the use of bone cement or through biological fixation.
1.4 Custom (designed explicitly for a single patient), revision, or constrained acetabular prostheses are not covered within the scope of this specification.
1.5 This specification does not cover the details for quality assurance, design control, production control contained in 21 CFR 820 (Quality System Regulation) and ISO 9001.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F2091 −01(Reapproved 2012)
Standard Specification for
Acetabular Prostheses
This standard is issued under the fixed designation F2091; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope F136 Specification for Wrought Titanium-6Aluminum-
4Vanadium ELI (Extra Low Interstitial)Alloy for Surgical
1.1 This specification covers acetabular resurfacing devices
Implant Applications (UNS R56401)
used to provide a functioning articulation between the bones of
F138 Specification for Wrought 18Chromium-14Nickel-
the acetabulum and the femur.
2.5Molybdenum Stainless Steel Bar andWire for Surgical
1.2 This specification is intended to provide basic descrip-
Implants (UNS S31673)
tions of materials and device geometry. Additionally, those
F562 Specification for Wrought 35Cobalt-35Nickel-
characteristics determined to be important to in vivo perfor-
20Chromium-10Molybdenum Alloy for Surgical Implant
mance of the device are defined.
Applications (UNS R30035)
F563 Specification for Wrought Cobalt-20Nickel-
1.3 Acetabular prostheses included within the scope of this
specification are intended for mechanical fixation between the 20Chromium-3.5Molybdenum-3.5Tungsten-5Iron Alloy
for Surgical Implant Applications (UNS R30563) (With-
prosthesis and host bone, by the use of bone cement or through
biological fixation. drawn 2005)
F601 Practice for Fluorescent Penetrant Inspection of Me-
1.4 Custom (designed explicitly for a single patient),
tallic Surgical Implants
revision, or constrained acetabular prostheses are not covered
F603 Specification for High-Purity Dense Aluminum Oxide
within the scope of this specification.
for Medical Application
1.5 This specification does not cover the details for quality
F629 Practice for Radiography of Cast Metallic Surgical
assurance, design control, production control contained in 21
Implants
CFR 820 (Quality System Regulation) and ISO 9001.
F648 Specification for Ultra-High-Molecular-Weight Poly-
ethylene Powder and Fabricated Form for Surgical Im-
2. Referenced Documents
plants
F745 Specification for 18Chromium-12.5Nickel-
2.1 ASTM Standards:
2.5Molybdenum Stainless Steel for Cast and Solution-
F67 Specification for Unalloyed Titanium, for Surgical Im-
Annealed Surgical Implant Applications (Withdrawn
plant Applications (UNS R50250, UNS R50400, UNS
2012)
R50550, UNS R50700)
F746 Test Method for Pitting or Crevice Corrosion of
F75 Specification for Cobalt-28 Chromium-6 Molybdenum
Metallic Surgical Implant Materials
Alloy Castings and Casting Alloy for Surgical Implants
F748 PracticeforSelectingGenericBiologicalTestMethods
(UNS R30075)
for Materials and Devices
F86 Practice for Surface Preparation and Marking of Metal-
F799 Specification for Cobalt-28Chromium-6Molybdenum
lic Surgical Implants
Alloy Forgings for Surgical Implants (UNS R31537,
F90 Specification for Wrought Cobalt-20Chromium-
R31538, R31539)
15Tungsten-10NickelAlloy for Surgical ImplantApplica-
tions (UNS R30605) F981 Practice for Assessment of Compatibility of Biomate-
rials for Surgical Implants with Respect to Effect of
Materials on Muscle and Bone
F983 Practice for Permanent Marking of Orthopaedic Im-
This specification is under the jurisdiction of ASTM Committee F04 on
Medical and Surgical Materials and Devices and is the direct responsibility of plant Components
Subcommittee F04.22 on Arthroplasty.
F1044 Test Method for Shear Testing of Calcium Phosphate
Current edition approved Jan. 15, 2012. Published January 2012. Originally
Coatings and Metallic Coatings
approved in 2001. Last previous edition approved in 2006 as F2091 – 01 (2006).
F1108 Specification for Titanium-6Aluminum-4Vanadium
DOI: 10.1520/F2091-01R11.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The last approved version of this historical standard is referenced on
the ASTM website. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F2091−01 (2012)
Alloy Castings for Surgical Implants (UNS R56406) elements; instruments for insertion, extraction, and so forth; or
F1147 Test Method for Tension Testing of Calcium Phos- for manufacturing purposes.
phate and Metallic Coatings
3.1.3 fixation element, n—any peg, spike, threadform, or
F1160 Test Method for Shear and Bending Fatigue Testing
other protrusion from the exterior surface of the shell intended
of Calcium Phosphate and Metallic Medical and Compos-
toincreasethesurfacecontactormechanicalinterlockbetween
ite Calcium Phosphate/Metallic Coatings
the component, the bonding agent, the natural acetabulum, or a
F1185 Specification for Composition of Hydroxylapatite for
combination thereof.
Surgical Implants
3.1.4 flange, n—rim extending from the entry diameter of
F1377 Specification for Cobalt-28Chromium-6Molybdenum
bearing element.
Powder for Coating of Orthopedic Implants (UNS
3.1.5 porous coating, n—a region on the exterior surface of
R30075)
the shell characterized by interconnecting subsurface pores,
F1472 Specification for Wrought Titanium-6Aluminum-
generally with volume porosity between 30 and 70 %, average
4VanadiumAlloy for Surgical ImplantApplications (UNS
pore size between 100 and 1000 µm, and a thickness between
R56400)
500 and 1500 µm. This porous layer may be manufactured
F1501 Test Method for Tension Testing of Calcium Phos-
directly into the device by casting or by various electro/
phate Coatings (Withdrawn 2000)
chemical/thermal/mechanical means, or applied as a coating of
F1537 Specification for Wrought Cobalt-28Chromium-
particles, beads, or mesh by processes such as sintering or
6Molybdenum Alloys for Surgical Implants (UNS
plasma spray.
R31537, UNS R31538, and UNS R31539)
F1580 Specification for Titanium and Titanium-6
3.1.6 radiographic marker, n—nonstructural, generally thin
Aluminum-4 Vanadium Alloy Powders for Coatings of wire, designed to be apparent on X-rays taken after placement
Surgical Implants
of implants that otherwise would be unapparent on such
F1714 GuideforGravimetricWearAssessmentofProsthetic X-rays.
Hip Designs in Simulator Devices
3.1.7 retention element, n—any ring, taper, wire, or other
F1820 Test Method for Determining the Forces for Disas-
protrusion or cavity from the interior surface of the shell or the
sembly of Modular Acetabular Devices
exterior surface of the bearing element that is intended to affix
F1978 Test Method for Measuring Abrasion Resistance of
the bearing element to the shell.
Metallic Thermal Spray Coatings by Using the Taber
3.1.8 shell, n—metal structure supporting the articulating
Abraser
surface material, and which may be fixed rigidly to the
F2033 Specification for Total Hip Joint Prosthesis and Hip
articulating surface or fixed such that it allows the articulating
Endoprosthesis Bearing Surfaces Made of Metallic,
surface to rotate or translate.
Ceramic, and Polymeric Materials
3.1.9 surface texturing, n—repetitive or random deviations
2.2 ISO Standards:
from the nominal surface that forms the three dimensional
ISO 5832 Implants for surgery—Metallic materials for sur-
4 topography of the surface.
gical implants
ISO 5834 Implants for surgery—Ultra high molecular 3.2 Dimensions of acetabular prostheses should be desig-
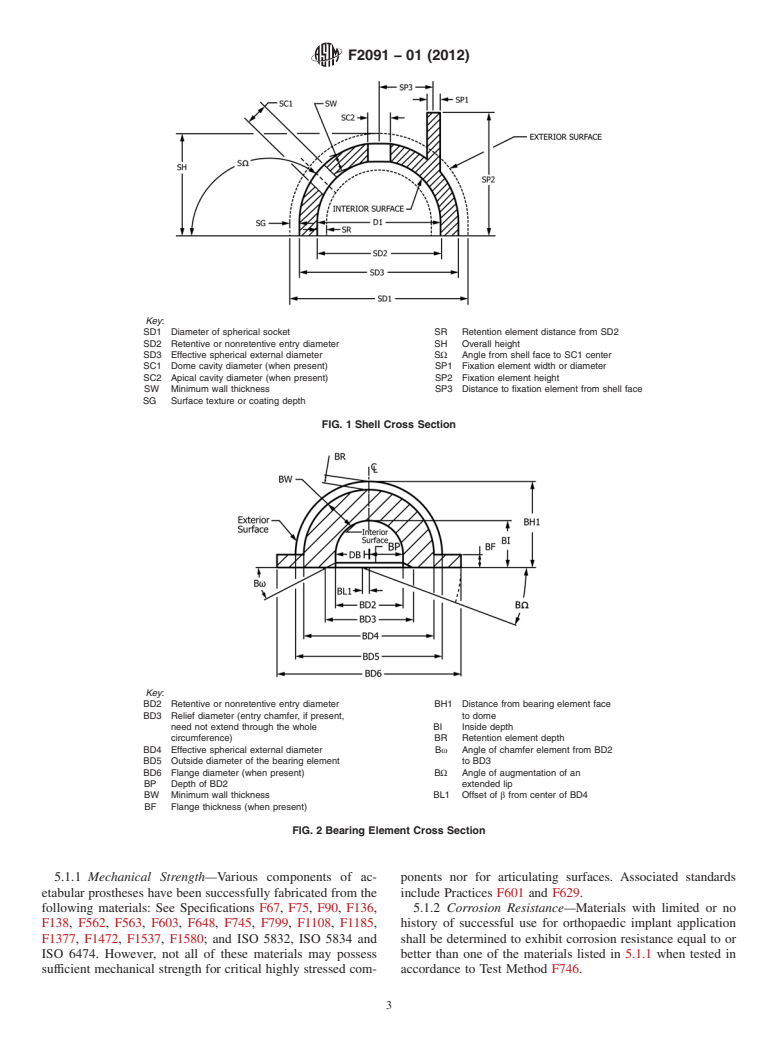
weight polyethylene nated in accordance with Figs. 1-3 or by an equally acceptable
ISO6474 Implantsforsurgery—Ceramicmaterialsbasedon and detailed method.
high purity alumina
NOTE 1—Figs. 1-3 are intended to be illustrative of typical acetabular
ISO 9001 Quality systems—Model for quality assurance in
prostheses and to designate dimensions, but representation of the compo-
design/development, production, installation, and servic-
nents does not otherwise form part of the standard.
ing
4. Types
2.3 Code of Federal Regulations:
4.1 Acetabular prostheses falling within the scope of this
21 CFR 820 Quality System Regulation
specification are of two types, as defined below. There are no
3. Terminology distinguishing features (for example, augmentation or lack
thereof,holes,andsoforth)thatwouldexemptanydevicefrom
3.1 Definitions:
any requirement of this specification.
3.1.1 bearing element, n—articulating surface element be-
4.1.1 Type I—Single-piece acetabular prostheses.
tween the femoral head and shell or bonding agent (bone
cement). NOTE 2—Specifications to both bearing elements and shell may apply.
3.1.2 cavity, n—any slot, cut, hole, or other feature within 4.1.2 Type II—Multipiece, modular structure prostheses.
the shell intended to accommodate modular adjunct fixation
5. Material
5.1 The choice of materials is understood to be a necessary,
Available from International Organization for Standardization (ISO), 1, ch. de
but not sufficient, assurance of function of the device made
la Voie-Creuse, CP 56, CH-1211 Geneva 20, Switzerland, http://www.iso.org.
fromthem.Alldevicesconformingtothisspecificationshallbe
Available from Standardization Documents Order Desk, DODSSP, Bldg. 4,
fabricated from materials with adequate mechanical strength
Section D, 700 Robbins Ave., Philadelphia, PA 19111-5098, http://
dodssp.daps.dla.mil. and durability, corrosion resistance, and biocompatibility.
F2091−01 (2012)
Key:
SD1 Diameter of spherical socket SR Retention element distance from SD2
SD2 Retentive or nonretentive entry diameter SH Overall height
SD3 Effective spherical external diameter SΩ Angle from shell face to SC1 center
SC1 Dome cavity diameter (when present) SP1 Fixation element width or diameter
SC2 Apical cavity diameter (when present) SP2 Fixation element height
SW Minimum wall thickness SP3 Distance to fixation element from shell face
SG Surface texture or coating depth
FIG. 1Shell Cross Section
Key:
BD2 Retentive or nonretentive entry diameter BH1 Distance from bearing element face
BD3 Relief diameter (entry chamfer, if present, to dome
need not extend through the whole BI Inside depth
circumference) BR Retention element depth
BD4 Effectiv
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.