ASTM D2386-15
(Test Method)Standard Test Method for Freezing Point of Aviation Fuels
Standard Test Method for Freezing Point of Aviation Fuels
SIGNIFICANCE AND USE
4.1 The freezing point of an aviation fuel is the lowest temperature at which the fuel remains free of solid hydrocarbon crystals that can restrict the flow of fuel through filters if present in the fuel system of the aircraft. The temperature of the fuel in the aircraft tank normally falls during flight depending on aircraft speed, altitude, and flight duration. The freezing point of the fuel must always be lower than the minimum operational tank temperature.
4.2 Freezing point is a requirement in Specifications D910 and D1655.
SCOPE
1.1 This test method covers the determination of the temperature below which solid hydrocarbon crystals may form in aviation turbine fuels and aviation gasoline.
Note 1: The interlaboratory program that generated the precisions for this test method did not include aviation gasoline.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 WARNING—Mercury has been designated by many regulatory agencies as a hazardous material that can cause central nervous system, kidney and liver damage. Mercury, or its vapor, may be hazardous to health and corrosive to materials. Caution should be taken when handling mercury and mercury containing products. See the applicable product Material Safety Data Sheet (MSDS) for details and EPA’s website—http://www.epa.gov/mercury/faq.htm—for additional information. Users should be aware that selling mercury and/or mercury containing products into your state or country may be prohibited by law.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific warning statements, see 5.4, Section 6, and 8.3.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D2386 − 15
Designation:16/15
Standard Test Method for
1
Freezing Point of Aviation Fuels
This standard is issued under the fixed designation D2386; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope* D1655Specification for Aviation Turbine Fuels
D3117Test Method for Wax Appearance Point of Distillate
1.1 This test method covers the determination of the tem-
3
Fuels (Withdrawn 2010)
perature below which solid hydrocarbon crystals may form in
D4057Practice for Manual Sampling of Petroleum and
aviation turbine fuels and aviation gasoline.
Petroleum Products
NOTE 1—The interlaboratory program that generated the precisions for
D4177Practice for Automatic Sampling of Petroleum and
this test method did not include aviation gasoline.
Petroleum Products
1.2 The values stated in SI units are to be regarded as
E1Specification for ASTM Liquid-in-Glass Thermometers
standard. No other units of measurement are included in this
E77Test Method for Inspection and Verification of Ther-
standard.
mometers
1.3 WARNING—Mercury has been designated by many
2.2 Energy Institute Standard:
4
regulatory agencies as a hazardous material that can cause
IP Standards for Petroleum and Its Products IP 16/15
central nervous system, kidney and liver damage. Mercury, or
its vapor, may be hazardous to health and corrosive to
3. Terminology
materials.Cautionshouldbetakenwhenhandlingmercuryand
3.1 Definitions of Terms Specific to This Standard:
mercury containing products. See the applicable product Ma-
3.1.1 freezing point, n—in aviation fuels, the fuel tempera-
terial Safety Data Sheet (MSDS) for details and EPA’s
ture at which solid hydrocarbon crystals, formed on cooling,
website—http://www.epa.gov/mercury/faq.htm—for addi-
disappear when the temperature of the fuel is allowed to rise
tional information. Users should be aware that selling mercury
under specified conditions of test.
and/or mercury containing products into your state or country
3.1.2 crystallization point, n—the temperature at which
may be prohibited by law.
crystals of hydrocarbons first appear when the test sample is
1.4 This standard does not purport to address all of the
cooled.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
4. Significance and Use
priate safety and health practices and determine the applica-
4.1 The freezing point of an aviation fuel is the lowest
bility of regulatory limitations prior to use. For specific
temperature at which the fuel remains free of solid hydrocar-
warning statements, see 5.4, Section 6, and 8.3.
bon crystals that can restrict the flow of fuel through filters if
2. Referenced Documents presentinthefuelsystemoftheaircraft.Thetemperatureofthe
2
fuel in the aircraft tank normally falls during flight depending
2.1 ASTM Standards:
on aircraft speed, altitude, and flight duration. The freezing
D910Specification for Leaded Aviation Gasolines
point of the fuel must always be lower than the minimum
operational tank temperature.
1
This test method is under the jurisdiction of ASTM Committee D02 on
4.2 Freezing point is a requirement in Specifications D910
Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of
and D1655.
Subcommittee D02.07 on Flow Properties.
Current edition approved June 1, 2015. Published June 2015. Originally
approved in 1965. Last previous edition approved in 2012 as D2386–06 (2012).
DOI: 10.1520/D2386-15.
2 3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or The last approved version of this historical standard is referenced on
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM www.astm.org.
4
Standards volume information, refer to the standard’s Document Summary page on Available from Energy Institute, 61 New Cavendish St., London, WIG 7AR,
the ASTM website. U.K., http://www.energyinst.org.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
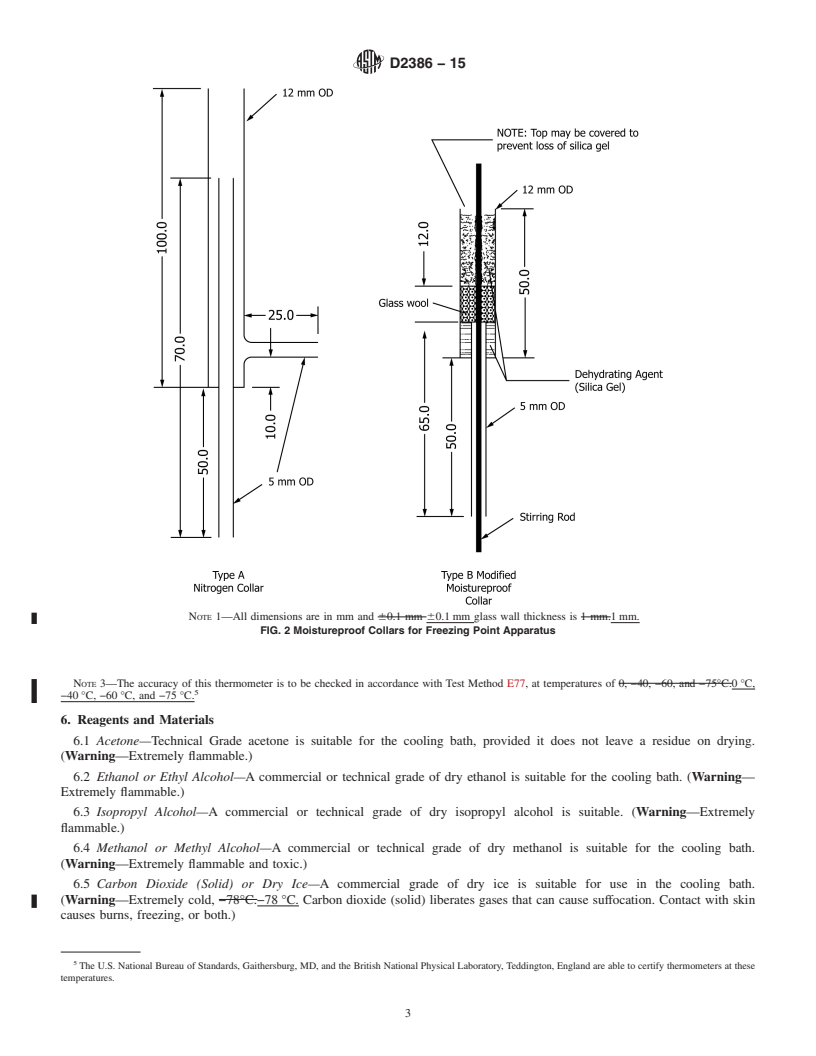
D2386 − 15
accordance with Test Method E77, at temperatures of 0°C, −40°C,
5. Apparatus
5
−60°C, and −75°C.
5.1 Jacketed Sample Tube—A double-walled, unsilvered
vessel, similar to a Dewar flask, the space between the inner
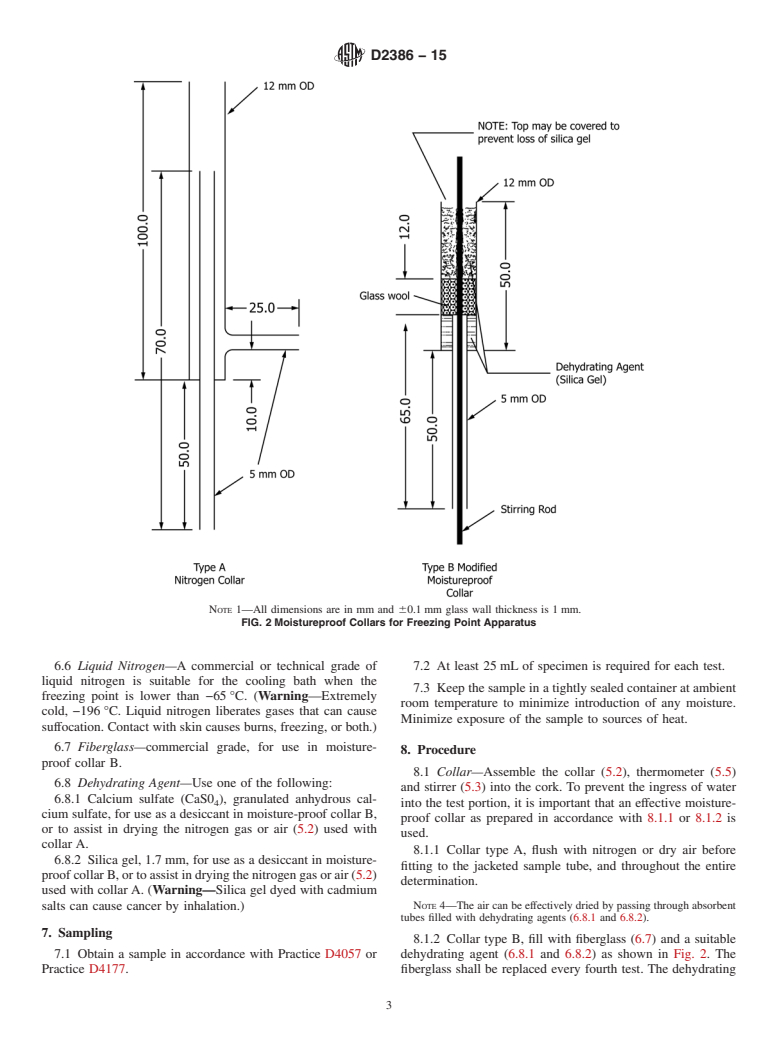
6. Reagents and Materials
and outer tube walls being filled at atmospheric pressure with
6.1 Acetone—Technical Grade acetone is suitable
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D2386 − 06 (Reapproved 2012) D2386 − 15
Designation: 16/15
Standard Test Method for
1
Freezing Point of Aviation Fuels
This standard is issued under the fixed designation D2386; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope*
1.1 This test method covers the determination of the temperature below which solid hydrocarbon crystals may form in aviation
turbine fuels and aviation gasoline.
NOTE 1—The interlaboratory program that generated the precisions for this test method did not include aviation gasoline.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 WARNING—Mercury has been designated by many regulatory agencies as a hazardous material that can cause central
nervous system, kidney and liver damage. Mercury, or its vapor, may be hazardous to health and corrosive to materials. Caution
should be taken when handling mercury and mercury containing products. See the applicable product Material Safety Data Sheet
(MSDS) for details and EPA’s website—http://www.epa.gov/mercury/faq.htm—for additional information. Users should be aware
that selling mercury and/or mercury containing products into your state or country may be prohibited by law.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use. For specific warning statements, see 5.4, Section 6, and 8.28.3.
2. Referenced Documents
2
2.1 ASTM Standards:
D910 Specification for Leaded Aviation Gasolines
D1655 Specification for Aviation Turbine Fuels
3
D3117 Test Method for Wax Appearance Point of Distillate Fuels (Withdrawn 2010)
D4057 Practice for Manual Sampling of Petroleum and Petroleum Products
D4177 Practice for Automatic Sampling of Petroleum and Petroleum Products
E1 Specification for ASTM Liquid-in-Glass Thermometers
E77 Test Method for Inspection and Verification of Thermometers
2.2 Energy Institute Standard:
4
IP Standards for Petroleum and Its Products, Part 1Products IP 16/15
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 freezing point, n—in aviation fuels, the fuel temperature at which solid hydrocarbon crystals, formed on cooling, disappear
when the temperature of the fuel is allowed to rise under specified conditions of test.
3.1.2 crystallization point, n—the temperature at which crystals of hydrocarbons first appear when the test sample is cooled.
1
This test method is under the jurisdiction of ASTM Committee D02 on Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of Subcommittee
D02.07 on Flow Properties.
Current edition approved April 15, 2012June 1, 2015. Published April 2012June 2015. Originally approved in 1965. Last previous edition approved in 20062012 as
D2386D2386 – 06 (2012).–06. DOI: 10.1520/D2386-06R12.10.1520/D2386-15.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
The last approved version of this historical standard is referenced on www.astm.org.
4
Available from Energy Institute, 61 New Cavendish St., London, WIG 7AR, U.K., http://www.energyinst.org.
*A Summary of Changes section appears at the end of this standard
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
D2386 − 15
4. Significance and Use
4.1 The freezing point of an aviation fuel is the lowest temperature at which the fuel remains free of solid hydrocarbon crystals
that can restrict the flow of fuel through filters if present in the fuel system of the aircraft. The temperature of the fuel in the aircraft
tank normally falls during flight depending on aircraft speed, altitude, and flight duration. The freezing point of the fuel must
always be lower than the minimum operational tank temperature.
4.2 Freezing po
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.