ASTM F2458-05(2010)
(Test Method)Standard Test Method for Wound Closure Strength of Tissue Adhesives and Sealants
Standard Test Method for Wound Closure Strength of Tissue Adhesives and Sealants
SIGNIFICANCE AND USE
Materials and devices that function at least in part by adhering to living tissues are finding increasing use in surgical procedures either as adjuncts to sutures and staples, or as frank replacements for those devices in a wide variety of medical procedures. While the nature and magnitude of the forces involved varies greatly with indication and with patient specific circumstances, all uses involve to some extent the ability of the material to resist imposed mechanical forces. Therefore, the mechanical properties of the materials, and in particular the adhesive properties, are important parameters in evaluating their fitness for use. In addition, the mechanical properties of a given adhesive composition can provide a useful means of determining product consistency for quality control or as a means for determining the effects of various surface treatments on the substrate prior to use of the device.
The complexity and variety of individual applications for tissue adhesive devices, even within a single indicated use (surgical procedure, which itself may vary depending on physical site and clinical intention) is such that the results of a single tensile strength test is not suitable for determining allowable design stresses without thorough analysis and understanding of the application, adhesive behaviors, and clinical indications.
This test method may be used for comparing adhesives or bonding processes for susceptibility to fatigue, mode of failure, and environmental changes, but such comparisons must be made with great caution since different adhesives may respond differently to varying conditions.
A correlation of the test method results with actual adhesive performance in live human tissue has not been established.
SCOPE
1.1 This test method covers a means for comparison of wound closure strength of tissue adhesives used to help secure the apposition of soft tissue. With the appropriate choice of substrate, it may also be used for purposes of quality control in the manufacture of medical devices used as tissue adhesives.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2458 − 05(Reapproved 2010)
Standard Test Method for
Wound Closure Strength of Tissue Adhesives and Sealants
This standard is issued under the fixed designation F2458; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.2.2 tissue sealant—a surface coating with adequate adhe-
sive strength to prevent leakage of body fluids.
1.1 This test method covers a means for comparison of
3.2.3 cohesive strength—internal strength of the adhesive.
wound closure strength of tissue adhesives used to help secure
the apposition of soft tissue. With the appropriate choice of
3.2.4 adhesive strength—the strength of the tissue adhesive/
substrate,itmayalsobeusedforpurposesofqualitycontrolin
substrate interface.
the manufacture of medical devices used as tissue adhesives.
3.2.5 cohesivefailure—failureoftheinternaladhesivebond.
1.2 The values stated in SI units are to be regarded as
3.2.6 adhesive failure—failure of the adhesive/substrate
standard. No other units of measurement are included in this
bond.
standard.
3.2.7 substrate failure—failure of the tissue substrate.
1.3 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the 4. Significance and Use
responsibility of the user of this standard to establish appro-
4.1 Materials and devices that function at least in part by
priate safety and health practices and determine the applica-
adhering to living tissues are finding increasing use in surgical
bility of regulatory limitations prior to use.
procedureseitherasadjunctstosuturesandstaples,orasfrank
replacements for those devices in a wide variety of medical
2. Referenced Documents
procedures. While the nature and magnitude of the forces
involvedvariesgreatlywithindicationandwithpatientspecific
2.1 ASTM Standards:
D907Terminology of Adhesives circumstances,allusesinvolvetosomeextenttheabilityofthe
material to resist imposed mechanical forces. Therefore, the
E4Practices for Force Verification of Testing Machines
mechanical properties of the materials, and in particular the
2.2 Other Document:
adhesive properties, are important parameters in evaluating
American Association of Tissue Banking,Standards for
3 their fitness for use. In addition, the mechanical properties of a
Tissue Banking
given adhesive composition can provide a useful means of
determining product consistency for quality control or as a
3. Terminology
meansfordeterminingtheeffectsofvarioussurfacetreatments
3.1 Many terms in this test method are defined inTerminol-
on the substrate prior to use of the device.
ogy D907.
4.2 The complexity and variety of individual applications
3.2 Definitions:
for tissue adhesive devices, even within a single indicated use
3.2.1 tissue adhesive—any material used as a medical de-
(surgical procedure, which itself may vary depending on
vice to help secure the apposition of two wound edges or
physical site and clinical intention) is such that the results of a
opposed soft tissues.
single tensile strength test is not suitable for determining
allowabledesignstresseswithoutthoroughanalysisandunder-
standing of the application, adhesive behaviors, and clinical
indications.
ThistestmethodisunderthejurisdictionofASTMCommitteeF04onMedical
andSurgicalMaterialsandDevicesandisthedirectresponsibilityofSubcommittee
4.3 This test method may be used for comparing adhesives
F04.15 on Material Test Methods.
or bonding processes for susceptibility to fatigue, mode of
Current edition approved June 1, 2010. Published September 2010. Originally
approved in 2005. Last previous edition approved in 2005 as F2458-05. DOI:
failure,andenvironmentalchanges,butsuchcomparisonsmust
10.1520/F2458-05R10.
be made with great caution since different adhesives may
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
respond differently to varying conditions.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
4.4 A correlation of the test method results with actual
the ASTM website.
adhesive performance in live human tissue has not been
Available from American Association of Tissue Banks (AATB), 1320 Old
Chain Bridge Rd., Suite 450, McLean, VA 22101. established.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F2458 − 05 (2010)
5. Apparatus 6.2 For Application Specific Testing—The grips of the test
machine must be able to hold the tissue without having the
5.1 Testing Machine—A testing machine of the constant-
tissue slip or be crushed by the grips. Some tissue (liver, lung)
rate-of-crosshead-movement type and comprising essentially
may not be suitable for this test.
the following:
6.2.1 The strength of any adhesive is highly dependent on
5.1.1 Fixed Member—A fixed or essentially stationary
the test substrate or adherend. For a specific application, the
member carrying one grip.
preferred substrate is freshly harvested tissue from the target
5.1.2 Movable Member—A movable member carrying a
organ of a domestic food animal. Tissue from bovine, porcine,
second grip.
orovineoriginispreferredduetowideavailabilityandthefact
5.1.3 Grips—Grips for holding the test specimen between
that relatively large samples of tissue can be harvested from a
the fixed member and the movable member of the testing
single source. Ideally, the tissue should be used within 24 h of
machine can be either the fixed or self-aligning type. Gripping
harvestandshouldbekeptbetween5and10°Cpriortotesting
pressure should be adjustable to prevent damage to the
if it cannot be used immediately after harvesting. Storage and
substrate and the use of sandpaper or plastic scrubbing pads
handling of tissue samples should be carried out according to
between the gripping surfaces and the substrate is recom-
the guidelines set forth in Standards forTissue Banking by the
mended to help prevent slippage.
AmericanAssociation of Tissue Banks. The specimens should
5.1.3.1 Fixed grips are rigidly attached to the fixed and
be brought to the test temperature or other prescribed tempera-
movable members of the testing machine. When this type of
ture (such as body temperature) prior to application of the
gripisused,extremecareshouldbetakentoensurethatthetest
adhesive.
specimen is inserted and clamped so that the long axis of the
6.2.2 Fixed tissue should not be used since it has been
test specimen coincides with the direction of pull through the
demonstrated that fixatives cause large alterations in the
centerline of the grip assembly.
mechanical properties of the tissue and it is probable that the
5.1.3.2 Self-aligning grips are attached to the fixed and
adhesive strength would be affected as well.
movablemembersofthetestingmachineinsuchamannerthat
6.2.3 If the target organ is of a size or geometry that does
they will move freely into alignment as soon as any load is
notallowfabricationoftestsamplesasshowninFig.1,atissue
applied so that the long axis of the test specimen will coincide
ofsimilaroriginbutlargersizeshouldbeused.Forexample,if
with the direction of the applied pull through the center line of
the intended indication is for anastomosis of small blood
the grip assembly. The specimens should be aligned as per-
vessels, a larger vessel should be substituted.
fectly as possible with the direction of pull so that no rotary
6.2.4 The thickness of the tissue sample should not exceed
motion that may induce slippage or damage to the sample will
5 mm.
occur in the grips; there is a limit to the amount of misalign-
ment self-aligning grips will accommodate. 6.3 For Quality Control Testing:
5.1.4 Drive Mechanism—A drive mechanism for imparting 6.3.1 For testing that is undertaken as part of a quality
to the movable member a uniform, controlled velocity with control process in the manufacturing of a tissue adhesive
respect to the stationary member, with this velocity to be device, the use of freshly harvested tissue is highly inconve-
regulated as specified in 8.3.
nient and may also lead to unacceptable variation in the test
5.1.5 Load Indicator—A suitable load-indicating mecha- results, especially if the failure occurs in the adherend (sub-
nism capable of showing the total tensile load carried by the
strate failure). Since the purpose of quality control testing is to
testspecimenwhenheldbythegrips.Thismechanismshallbe demonstrate consistency in the device, substitution of a model
essentially free of inertia lag at the specified rate of testing and
substrate is preferred so long as it is demonstrated that the
shall indicate the load with an accuracy of 61% of the adhesive bonds to the adherend. If the test is intended to
indicated value, or better. The accuracy of the testing machine
generate data on the cohesive strength of the device, any
shall be verified in accordance with Practices E4. metallic or polymeric material is acceptable so long as it has
beendemonstratedthattheadhesivebondstotheadherendand
5.2 Temperature-controlling Equipment—Capable of main-
that failure is substantially cohesive (>90% by area) and not
taining the test temperature to 62°C. If ambient laboratory
adhesive. For adhesive quality control testing, it is recom-
conditions are employed, the same degree of control is re-
mended that test results for any substrate of non-biological or
quired.
fixed tissue origin be correlated to testing previously done on
fresh tissue substrates prior to acceptance of the procedure.
6. Test Substrate
6.1 For Comparative Testing—Either fresh or frozen split
7. Test Specimen
thickness porcine skin graft may be used.
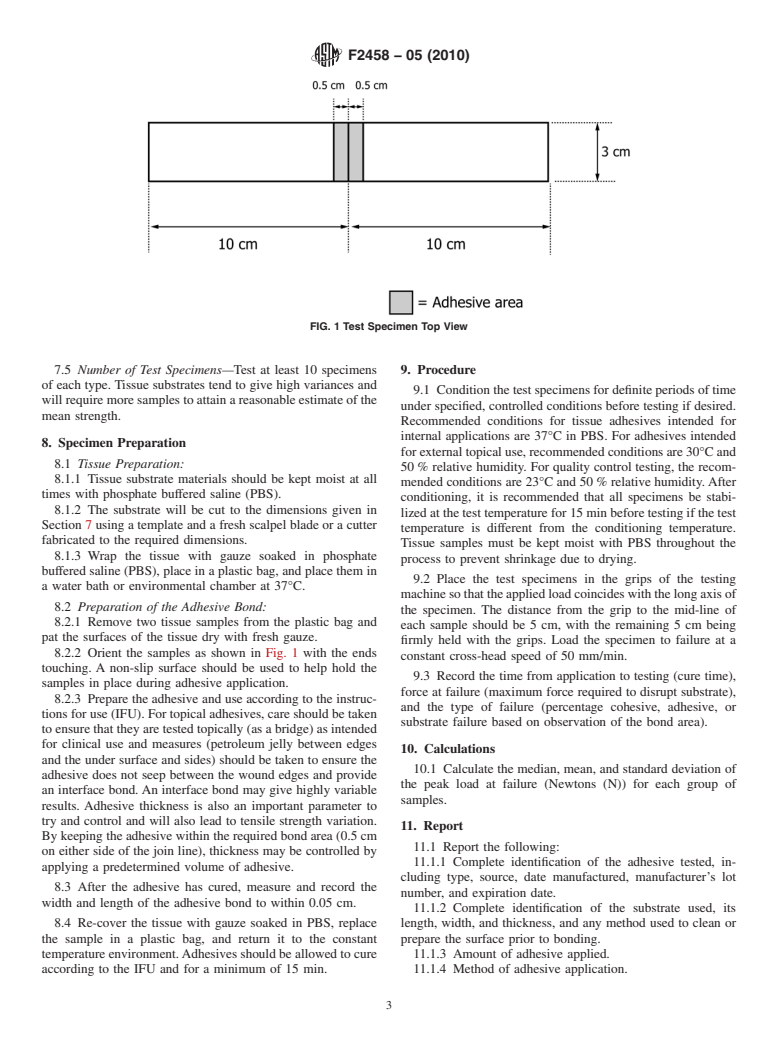
7.1 The wound strength test specimen is shown in Fig. 1.
6.1.1 Frozen split thickness porcine skin that has been
Two substrate samples are required for each test specimen.
aseptically prepared is available commercially and is preferred
due to ease of use and the potential for more consistent
7.2 Atemplate of the correct dimensions should be used.A
properties.Itshouldbethawedaccordingtothemanufacturer’s
sharp scalpel or similar device
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.