ASTM F1875-98(2009)
(Practice)Standard Practice for Fretting Corrosion Testing of Modular Implant Interfaces: Hip Femoral Head-bore and Cone Taper Interface
Standard Practice for Fretting Corrosion Testing of Modular Implant Interfaces: Hip Femoral Head-bore and Cone Taper Interface
SIGNIFICANCE AND USE
The modular interfaces of total joint prostheses are subjected to micromotion that could result in fretting and corrosion. The release of corrosion products and particulate debris could stimulate adverse biological reactions, as well as lead to accelerated wear at the articulation interface. Methods to assess the stability and corrosion resistance of the modular interfaces, therefore, are an essential component of device testing.
Long-term in-vitro testing is essential to produce damage and debris from fretting of a modular interface (4,5). The use of proteinaceous solutions is recommended to best simulate the in-vivo environment.
Short-term tests often can be useful in evaluations of differences in design during device development (1-4). The electrochemical methods provide semiquantitative measures of fretting corrosion rates. The relative contributions of mechanical and electrochemical processes to the total corrosion and particulate release phenomena, however, have not been established; therefore, these tests should not be utilized to compare the effects of changes in material combinations, but rather be utilized to evaluate design changes of bore (head) and cone (stem) components.
These tests are recommended for evaluating the fretting wear and corrosion of modular interfaces of hip femoral head and stem components. Similar methods may be applied to other modular interfaces where fretting corrosion is of concern.
These methods are recommended for comparative evaluation of the fretting wear and corrosion of new materials, coatings, or designs, or a combination thereof, under consideration for hip femoral head and neck modular interfaces. Components for testing may be those of a manufactured modular hip device (finished product) or sample coupons, which are designed and manufactured for simulation of the head, taper, and neck region of a modular hip device.
SCOPE
1.1 This practice describes the testing, analytical, and characterization methods for evaluating the mechanical stability of the bore and cone interface of the head and stem junction of modular hip implants subjected to cyclic loading by measurements of fretting corrosion (1-5). Two test methods described are as follows:
1.1.1 Method I—The primary purpose of this method is to provide a uniform set of guidelines for long-term testing to determine the amount of damage by measurement of the production of corrosion products and particulate debris from fretting and fretting corrosion. Damage is also assessed by characterization of the damage to the bore and cone surfaces (4, 5).
1.1.2 Methods II—This method provides for short-term electrochemical evaluation of the fretting corrosion of the modular interface. It is not the intent of this method to produce damage nor particulate debris but rather to provide a rapid method for qualitative assessment of design changes which do not include material changes (1-4).
1.2 This practice does not provide for judgment or prediction of in-vivo implant performance, but rather provides for a uniform set of guidelines for evaluating relative differences in performance between differing implant designs, constructs, or materials with performance defined in the context of the amount of fretting and fretting corrosion. Also, this practice should permit direct comparison of fretting corrosion data between independent research groups, and thus provide for building of a data base on modular implant performance.
1.3 This practice provides for comparative testing of manufactured hip femoral heads and stems and for coupon type specimen testing where the male taper portion of the modular junction does not include the entire hip implant, with the taper portion of the coupon identical in design, manufacturing, and materials to the taper of the final hip implant (4,5).
1.4 Method I of this practice permits simultaneous evaluation of the fatigue strength of a femoral hip stem (in accordance with P...
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F1875 − 98(Reapproved 2009)
Standard Practice for
Fretting Corrosion Testing of Modular Implant Interfaces:
Hip Femoral Head-Bore and Cone Taper Interface
This standard is issued under the fixed designation F1875; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope portion of the coupon identical in design, manufacturing, and
materials to the taper of the final hip implant (4,5).
1.1 This practice describes the testing, analytical, and char-
acterization methods for evaluating the mechanical stability of 1.4 Method I of this practice permits simultaneous evalua-
the bore and cone interface of the head and stem junction of tionofthefatiguestrengthofafemoralhipstem(inaccordance
modular hip implants subjected to cyclic loading by measure- with Practice F1440) and the mechanical stability and debris
ments of fretting corrosion (1-5). Two test methods described generated by fretting and fretting corrosion of the modular
are as follows: interface.
1.1.1 Method I—The primary purpose of this method is to
1.5 The general concepts and methodologies described in
provide a uniform set of guidelines for long-term testing to
this practice could be applied to the study of other modular
determine the amount of damage by measurement of the
interfaces in total joint prostheses.
production of corrosion products and particulate debris from
1.6 The values stated in SI units are to be regarded as
fretting and fretting corrosion. Damage is also assessed by
standard. No other units of measurement are included in this
characterization of the damage to the bore and cone surfaces
standard.
(4, 5).
1.7 This standard may involve hazardous materials,
1.1.2 Methods II—This method provides for short-term
electrochemical evaluation of the fretting corrosion of the operations, and equipment. This standard does not purport to
address all of the safety concerns, if any, associated with its
modular interface. It is not the intent of this method to produce
damage nor particulate debris but rather to provide a rapid use. It is the responsibility of the user of this standard to
establish appropriate safety and health practices and deter-
method for qualitative assessment of design changes which do
not include material changes (1-4). mine the applicability of regulatory limitations prior to use.
1.2 This practice does not provide for judgment or predic-
2. Referenced Documents
tion of in-vivo implant performance, but rather provides for a
2.1 ASTM Standards:
uniform set of guidelines for evaluating relative differences in
E4 Practices for Force Verification of Testing Machines
performance between differing implant designs, constructs, or
E466 Practice for Conducting Force Controlled Constant
materials with performance defined in the context of the
Amplitude Axial Fatigue Tests of Metallic Materials
amount of fretting and fretting corrosion. Also, this practice
E467 Practice for Verification of Constant Amplitude Dy-
should permit direct comparison of fretting corrosion data
namic Forces in an Axial Fatigue Testing System
between independent research groups, and thus provide for
F561 Practice for Retrieval and Analysis of Medical
building of a data base on modular implant performance.
Devices, and Associated Tissues and Fluids
1.3 This practice provides for comparative testing of manu-
F746 Test Method for Pitting or Crevice Corrosion of
factured hip femoral heads and stems and for coupon type
Metallic Surgical Implant Materials
specimen testing where the male taper portion of the modular
F897 Test Method for Measuring Fretting Corrosion of
junction does not include the entire hip implant, with the taper
Osteosynthesis Plates and Screws
F1440 Practice for Cyclic Fatigue Testing of Metallic
Stemmed HipArthroplasty Femoral Components Without
ThispracticeisunderthejurisdictionofASTMCommitteeF04onMedicaland
Torsion
Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.15 on Material Test Methods
Current edition approved Dec. 1, 2009. Published December 2009. Originally
approved in 1998. Last previous edition approved in 2004 as F1875 – 98(2004). For referenced ASTM standards, visit the ASTM website, www.astm.org, or
DOI: 10.1520/F1875-98R09. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
The bold face numbers in parentheses refers to the list of references at the end Standards volume information, refer to the standard’s Document Summary page on
of this standard. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F1875 − 98 (Reapproved 2009)
F1636 SpecificationforBoresandConesforModularFemo- 3.1.10 wear, n—damage to a solid surface, generally involv-
ral Heads (Withdrawn 2001) ingprogressivelossofmaterial,duetorelativemotionbetween
G3 Practice for Conventions Applicable to Electrochemical that surface and a contacting substance or substances.
Measurements in Corrosion Testing
4. Summary of Test Method
G5 Reference Test Method for Making Potentiostatic and
4.1 Method I—The femoral stem and head components, or
Potentiodynamic Anodic Polarization Measurements
coupons to simulate head-taper-neck geometry, are loaded
G15 Terminology Relating to Corrosion and CorrosionTest-
cyclically in a manner similar to that described in Practice
ing (Withdrawn 2010)
F1440. The head neck junction is exposed to a saline or
G40 Terminology Relating to Wear and Erosion
proteinaceous solution, either by immersion of the entire
G61 Test Method for Conducting Cyclic Potentiodynamic
device, or with a fluid containing envelope. The cyclic load is
Polarization Measurements for Localized Corrosion Sus-
applied for a minimum of 10 million cycles.At the conclusion
ceptibility of Iron-, Nickel-, or Cobalt-Based Alloys
of testing, the isolated fluid is withdrawn for chemical analysis
G102 Practice for Calculation of Corrosion Rates and Re-
for total elemental level, and characterization of particulate
lated Information from Electrochemical Measurements
debris. The taper interface is subsequently disengaged and the
2.2 ISO Standards:
surfaces inspected for fretting wear and corrosion using optical
ISO 7206-7 Endurance Performance of Stemmed Femoral
microscopy and scanning electron microscopy. The output of
Components Without Application of Torsion
thesemethodsisaquantitativemeasureoftotalelementallevel
3. Terminology
andaqualitativeevaluationofdamageofthemodularinterface
caused by fretting wear and corrosion.
3.1 Definitions:
3.1.1 corrosive wear, n—wear in which chemical or electro-
4.2 Method II—A coupon similar to that used in Method I,
chemical reaction with the environment is significant.
or an entire femoral stem and head construct, may be mounted
3.1.2 coverage, n—the length, parallel to the taper surface, in an inverted position in a test chamber. The chamber is filled
withanelectrolytesolutiontoalevelsufficienttosubmergethe
that the bore and cone interfaces are in contact.
bore and cone interface and a small portion of the exposed
3.1.3 crevice corrosion, n—localized corrosion of a metal
neck. The area of contact and articulation between the ball and
surface at, or immediately adjacent to, an area that is shielded
the test apparatus is isolated from the electrolyte, either by
from full exposure to the environment because of close
being above the fill level, or with an elastomeric seal used to
proximity between the metal and the surface of another
isolate the bottom of the test chamber.
material.
4.2.1 Procedure A—A saturated calomel electrode with a
3.1.4 external circuit, n—the wires, connectors, measuring
luggin probe is used as a reference electrode to measure
devices, current sources, and so forth that are used to bring
changes in the corrosion potential with an electrometer. A
about or measure the desired electrical conditions within the
counter electrode also may be employed and the polarization
test cell.
characteristics measured with a potentiostat.
3.1.5 femoral head neck extension, n—a distance parallel to
4.2.2 Procedure B—Alargesurfaceareacounterelectrodeis
the taper axis, from the nominal neck offset length (k)as
immersed in the solution to simulate the area of the stem. A
defined in Specification F1636, and the center of the head.
zero-resistance ammeter is connected between the test device
Such variants from the nominal length are used to adjust for
and the counter electrode. The difference in current, thus
resection level, leg length, and so forth. A positive neck
measured prior to and during cyclic loading, represents the
extension equates to the center of the head being located
fretting corrosion current flowing between the modular inter-
further away from the stem.
face (anode) and the metal sheet (cathode).
3.1.6 fretting, n—small amplitude oscillatory motion, usu-
5. Significance and Use
ally tangential, between two solid surfaces in contact.
5.1 The modular interfaces of total joint prostheses are
3.1.7 fretting corrosion, n—the deterioration at the interface
subjected to micromotion that could result in fretting and
between contacting surfaces as the result of corrosion and
corrosion. The release of corrosion products and particulate
slight oscillatory slip between the two surfaces.
debris could stimulate adverse biological reactions, as well as
3.1.8 fretting wear, n—wear arising as a result of fretting.
lead to accelerated wear at the articulation interface. Methods
3.1.9 total elemental level, n—the total weight of particulate to assess the stability and corrosion resistance of the modular
matter and corrosion ions generated by fretting wear and interfaces, therefore, are an essential component of device
fretting corrosion. Most analytical techniques are unable to testing.
accurately differentiate between ions and particulates, and
5.2 Long-term in-vitro testing is essential to produce dam-
therefore, total elemental level refers to all matter and corro-
age and debris from fretting of a modular interface (4,5). The
sion products released by fretting wear and corrosion.
useofproteinaceoussolutionsisrecommendedtobestsimulate
the in-vivo environment.
The last approved version of this historical standard is referenced on
5.3 Short-term tests often can be useful in evaluations of
www.astm.org.
differences in design during device development (1-4). The
Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
4th Floor, New York, NY 10036. electrochemical methods provide semiquantitative measures of
F1875 − 98 (Reapproved 2009)
fretting corrosion rates. The relative contributions of mechani- shown in Fig. 2. Coupon samples may be set up as shown in
cal and electrochemical processes to the total corrosion and Fig. 1, or in an inverted orientation.
particulate release phenomena, however, have not been estab-
6.4 Environmental Containment, Method I—The prosthesis
lished; therefore, these tests should not be utilized to compare
may be placed in an environmental chamber, which is filled
the effects of changes in material combinations, but rather be
with the appropriate fluid. Care should be taken to ensure that
utilized to evaluate design changes of bore (head) and cone
the contact area between the head and the low friction thrust
(stem) components.
bearing is not exposed to the electrolyte solution. The modular
5.4 These tests are recommended for evaluating the fretting interface of the prostheses or coupon samples also may be
wear and corrosion of modular interfaces of hip femoral head enclosed in an elastomeric sleeve, which contains the electro-
andstemcomponents.Similarmethodsmaybeappliedtoother lyte. The materials used for such isolation must be nonreactive
modular interfaces where fretting corrosion is of concern. and capable of retaining the fluid environment, (that is, prevent
leakage), throughout the course of testing. The volume of the
5.5 These methods are recommended for comparative
chamber shall be between 5 and 100 mL.
evaluation of the fretting wear and corrosion of new materials,
coatings, or designs, or a combination thereof, under consid-
NOTE 1—The use of small fluid volumes with the sleeve containment
method may not produce as much fretting corrosion as full prosthesis
eration for hip femoral head and neck modular interfaces.
exposure, due to the reduced surface area of the cathodic metal exposed.
Components for testing may be those of a manufactured
modular hip device (finished product) or sample coupons, 6.5 Environmental Chamber, Method II—The chamber shall
which are designed and manufactured for simulation of the be filled with electrolyte so as to submerge the modular
head, taper, and neck region of a modular hip device. interface.An elastomeric seal is used to isolate the contact area
between the head and the load application surface. Similar
6. Apparatus
seals should be employed for coupon sample testing. For
couponsorientedasshowninFig.1,thechamberfilllevelshall
6.1 Testing Machines—The action of the machine should be
bekeptbelowthearticulationbetweentheheadandtheloading
analyzedthereaftertoensurethatthedesiredformandperiodic
apparatus.
force amplitude is maintained for the duration of the test (see
Practice E467). The test machine should have a load monitor-
6.6 Counter and Reference Electrodes, Method II—Acoun-
ing system, such as the transducer mounted in line with the
ter electrode is included in the external circuit of Method II to
specimen. The loads should be monitored continuously in the
act as a cathode for measurement of corrosion currents. A
early stages of the test and periodically thereafter to ensure the
reference electrode is employed for measurement of the
desired load cycle is maintained. The varying load as deter-
corrosion potential of the specimen.
minedbysuitabledynamicverificationshouldbemaintainedat
6.6.1 Method II, Procedure A—The counter electrode and
all times to within 62 % of the maximum force being used in
saturated calomel electrode (SCE) shall be employed in accor-
accordance with Practices E4 and E466.
dance with Test Methods G5 and G61.
6.6.2 Method II, Procedure B—The counter electrode is
6.2 Specimen Mounting Devices, Method I—Modular hip
used to simulate the surface area of the femoral stem. It should
and stem components shall be set up as described in Practices
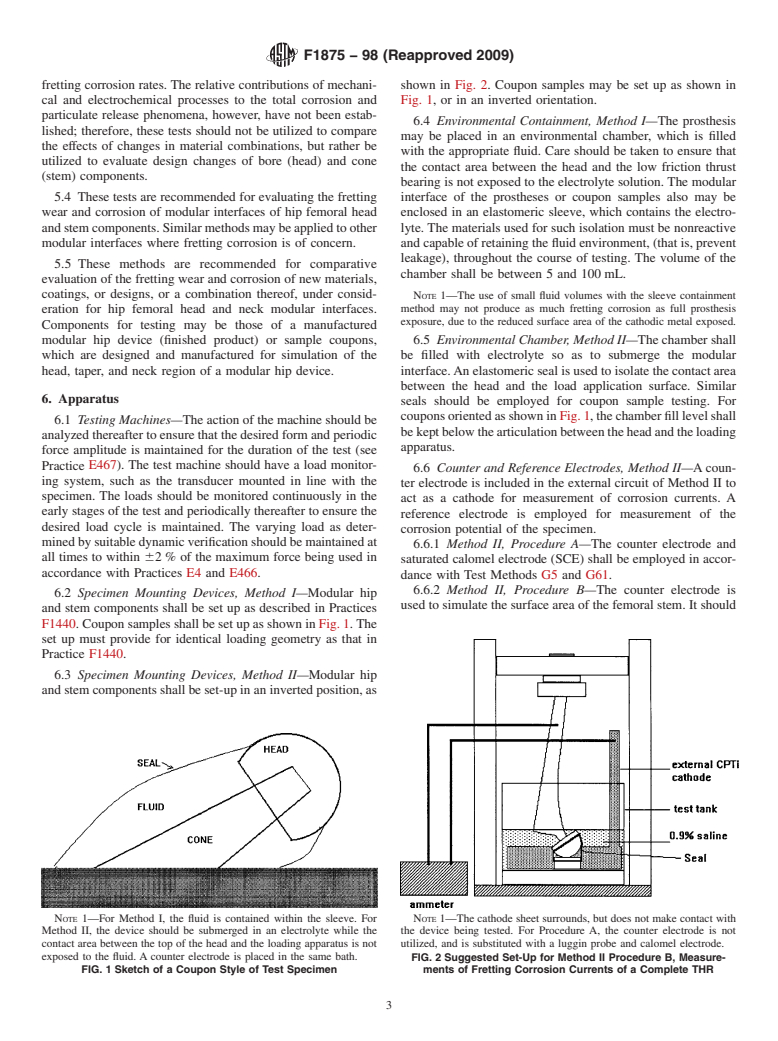
F1440. Coupon samples shall be set up as shown in Fig. 1.The
set up must provide for identical loading geometry as that in
Practice F1440.
6.3
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.