ASTM G108-94(2010)
(Test Method)Standard Test Method for Electrochemical Reactivation (EPR) for Detecting Sensitization of AISI Type 304 and 304L Stainless Steels
Standard Test Method for Electrochemical Reactivation (EPR) for Detecting Sensitization of AISI Type 304 and 304L Stainless Steels
SIGNIFICANCE AND USE
This test method describes an EPR test method for quantitatively determining the relative degree of sensitization in AISI Type 304 and 304L stainless steels. The EPR test has found wide use as a means to provide a numerical level of sensitization in studies of the effects of sensitization on intergranular corrosion and intergranular stress corrosion cracking behavior. The results of this test method correlate with other test methods (for example, Practices A262 and Test Methods G28) that are commonly used to assess sensitization in stainless steels.
The EPR test can also be used for product acceptance, service evaluation, regulatory statutes, and manufacturing controls providing that both the supplier and user have agreed upon appropriate acceptance criteria and a sensitizing treatment. The test is not intended for design purposes since the test conditions accelerate corrosion in a manner that does not simulate any actual service environment.
The EPR test involves the measurement of the amount of charge resulting from the corrosion of the chromium-depleted regions surrounding the precipitated chromium carbide particles. Most of these particles in a sensitized microstructure are located at the grain boundaries. However, discrete particles located within grains (referred to as intragranular precipitates) will also contribute to the total measured charge. (See Fig. 2.) Therefore, it is important to examine the alloy microstructure following an EPR test to determine the relative proportion of corrosion sites associated with intergranular versus intragranular precipitates. Sites of intergranular attack will appear similar to grain boundary ditching as defined in Practice A of Practices A262.
Note—The calculation of Pa is based on the assumptions illustrated at left. Mild cases of sensitization usually result in a combination of intergranular attack and pitting as illustrated at right (7). FIG. 2 Schematic Microstructures After EPR Testing
SCOPE
1.1 This test method covers a laboratory procedure for conducting an electrochemical reactivation (EPR) test on AISI Type 304 and 304L (UNS No. S30400 and S30403, respectively) stainless steels. This test method can provide a nondestructive means of quantifying the degree of sensitization in these steels (1, 2, 3). This test method has found wide acceptance in studies of the effects of sensitization on intergranular corrosion and intergranular stress corrosion cracking behavior (see Terminology G15). The EPR technique has been successfully used to evaluate other stainless steels and nickel base alloys (4), but the test conditions and evaluation criteria used were modified in each case from those cited in this test method.
1.2 The values stated in SI units are to be regarded as the standard. The inch-pound units given in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: G108 − 94(Reapproved 2010)
Standard Test Method for
Electrochemical Reactivation (EPR) for Detecting
Sensitization of AISI Type 304 and 304L Stainless Steels
This standard is issued under the fixed designation G108; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope E112Test Methods for Determining Average Grain Size
G1Practice for Preparing, Cleaning, and Evaluating Corro-
1.1 This test method covers a laboratory procedure for
sion Test Specimens
conducting an electrochemical reactivation (EPR) test onAISI
G3Practice for Conventions Applicable to Electrochemical
Type 304 and 304L (UNS No. S30400 and S30403, respec-
Measurements in Corrosion Testing
tively) stainless steels. This test method can provide a nonde-
G5Reference Test Method for Making Potentiodynamic
structive means of quantifying the degree of sensitization in
2 Anodic Polarization Measurements
these steels (1, 2, 3). This test method has found wide
G15TerminologyRelatingtoCorrosionandCorrosionTest-
acceptance in studies of the effects of sensitization on inter-
ing (Withdrawn 2010)
granular corrosion and intergranular stress corrosion cracking
G28Test Methods for Detecting Susceptibility to Inter-
behavior (seeTerminology G15).The EPR technique has been
granular Corrosion in Wrought, Nickel-Rich, Chromium-
successfully used to evaluate other stainless steels and nickel
Bearing Alloys
base alloys (4), but the test conditions and evaluation criteria
G61Test Method for Conducting Cyclic Potentiodynamic
used were modified in each case from those cited in this test
Polarization Measurements for Localized Corrosion Sus-
method.
ceptibility of Iron-, Nickel-, or Cobalt-Based Alloys
1.2 The values stated in SI units are to be regarded as the
standard. The inch-pound units given in parentheses are for
3. Terminology
information only.
3.1 Definitions of Terms Specific to This Standard:
1.3 This standard does not purport to address all of the
3.1.1 integrated charge (Q)—the charge measured, in
safety concerns, if any, associated with its use. It is the
couloumbs,duringreactivationasgivenbythetimeintegralof
responsibility of the user of this standard to establish appro-
current density below the reactivation peak of the curve.
priate safety and health practices and determine the applica-
3.1.2 maximum anodic current density (I )—the current
r
bility of regulatory limitations prior to use.
density measured at the peak of the anodic curve during
reactivation.
2. Referenced Documents
3.1.3 normalized charge (P )—the integrated current nor-
a
2.1 ASTM Standards:
malized to the specimen size and grain size. P represents the
a
A262Practices for Detecting Susceptibility to Intergranular
charge (in coulombs/cm ) of the grain-boundary area. The
Attack in Austenitic Stainless Steels
method for calculating P is given in 9.2.
a
D1193Specification for Reagent Water
3.1.4 reactivation—in the electrochemical reactivation
E3Guide for Preparation of Metallographic Specimens
(EPR) test, the potential sweep from the passivation potential
E7Terminology Relating to Metallography
returning to the corrosion potential.
3.1.5 scan rate—the rate at which the electrical potential
applied to a specimen in a polarization test is changed.
This test method is under the jurisdiction of ASTM Committee G01 on
Corrosion of Metals and is the direct responsibility of Subcommittee G01.11 on
Electrochemical Measurements in Corrosion Testing.
4. Summary of Test Method
Current edition approved May 1, 2010. Published May 2010. Originally
ε1
approved in 1992. Last previous edition approved in 2004 as G108–94(2004) . 4.1 The EPR test is accomplished by a potentiodynamic
DOI: 10.1520/G0108-94R10.
sweepfromthepassivetotheactiveregionsofelectrochemical
The boldface numbers in parentheses refer to a list of references at the end of
potentialsinaprocessreferredtoasreactivation.TheEPRtest
this standard.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The last approved version of this historical standard is referenced on
the ASTM website. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G108 − 94 (2010)
measurestheamountofchargeassociatedwiththecorrosionof activated and show higher Q and I values than solution
r
the chromium-depleted regions surrounding chromium carbide annealed steels, that are not susceptible to intergranular corro-
precipitated particles. Most of these particles in a sensitized sion. The value Q is normalized for both specimen size and
microstructure are located at grain boundaries (see Terminol- grainsize.Thevaluenormalizedinthisfashioniscalled P and
a
ogyE7).Discreteparticleslocatedwithinthegrain(referredto represents the charge (in units of coulombs) per unit grain-
as intragranular precipitates) will also contribute to the total boundary area. This normalization permits direct comparisons
measured charge. Therefore, it is important to examine the of different heats of material that exhibit different Q values
alloy microstructure following an EPR test, to determine the solely as a result of differences in grain size.
relativeproportionofcorrosionsiteassociatedwithintergranu-
lar versus intragranular precipitates. 5. Significance and Use
4.2 The chromium-depleted zones around carbide precipi- 5.1 This test method describes an EPR test method for
tates in sensitized steels are particularly susceptible to corro- quantitatively determining the relative degree of sensitization
sion in oxidizing acid solutions. Corrosion at chromium- in AISI Type 304 and 304L stainless steels. The EPR test has
depleted grain boundary sites causes a rapid rise in the current found wide use as a means to provide a numerical level of
densitywhentheelectrochemicalpotentialischangedfromthe sensitization in studies of the effects of sensitization on
passive to the active region.
intergranular corrosion and intergranular stress corrosion
cracking behavior. The results of this test method correlate
4.3 Asensitized steel produces a curve similar to the active
with other test methods (for example, Practices A262 and Test
portion of the polarization curve during the reactivation from
Methods G28) that are commonly used to assess sensitization
thepassiveregionbacktotherestpotential(E )asshownin
corr
in stainless steels.
Fig. 1. A nonsensitized (solution annealed) steel polarized
under the conditions given in this test method will produce a 5.2 The EPR test can also be used for product acceptance,
curve with lower current densities than a sensitized steel.
service evaluation, regulatory statutes, and manufacturing
controls providing that both the supplier and user have agreed
4.4 The EPR test results are readily reproducible, as long as
upon appropriate acceptance criteria and a sensitizing treat-
the electrolyte temperature, electrolyte composition, and scan
ment.Thetestisnotintendedfordesignpurposessincethetest
rate are carefully controlled. The EPR test is significantly
conditions accelerate corrosion in a manner that does not
affected by the composition, thermomechanical condition and
simulate any actual service environment.
surface finish of the specimen as well as the presence of
non-metallic inclusions, that result in pitting of the etched 5.3 The EPR test involves the measurement of the amount
microstructure. of charge resulting from the corrosion of the chromium-
depleted regions surrounding the precipitated chromium car-
NOTE 1—Various cutting and grinding operations can promote sensiti-
bide particles. Most of these particles in a sensitized micro-
zation of Type 304 (5). Superficial carbide precipitation can occur during
structurearelocatedatthegrainboundaries.However,discrete
cutting and grinding or during subsequent low temperature heat
treatments, such as 24 h at 500°C.
particles located within grains (referred to as intragranular
precipitates) will also contribute to the total measured charge.
4.5 The criteria used to distinguish between sensitized and
(See Fig. 2.) Therefore, it is important to examine the alloy
solution annealed samples are the activation charge density, Q
microstructure following an EPR test to determine the relative
(given by the time integral of current density below the
proportion of corrosion sites associated with intergranular
reactivationpeakofthecurve),orthemaximumanodiccurrent
versus intragranular precipitates. Sites of intergranular attack
density, I , in the active state. Sensitized steels are easily
r
will appear similar to grain boundary ditching as defined in
Practice A of Practices A262.
NOTE 1—The calculation of P is based on the assumptions illustrated
a
at left. Mild cases of sensitization usually result in a combination of
intergranular attack and pitting as illustrated at right (6).
FIG. 1 Schematic EPR Curves for Sensitized and Solutionized
AISI Type 304 Stainless Steel FIG. 2 Schematic Microstructures After EPR Testing
G108 − 94 (2010)
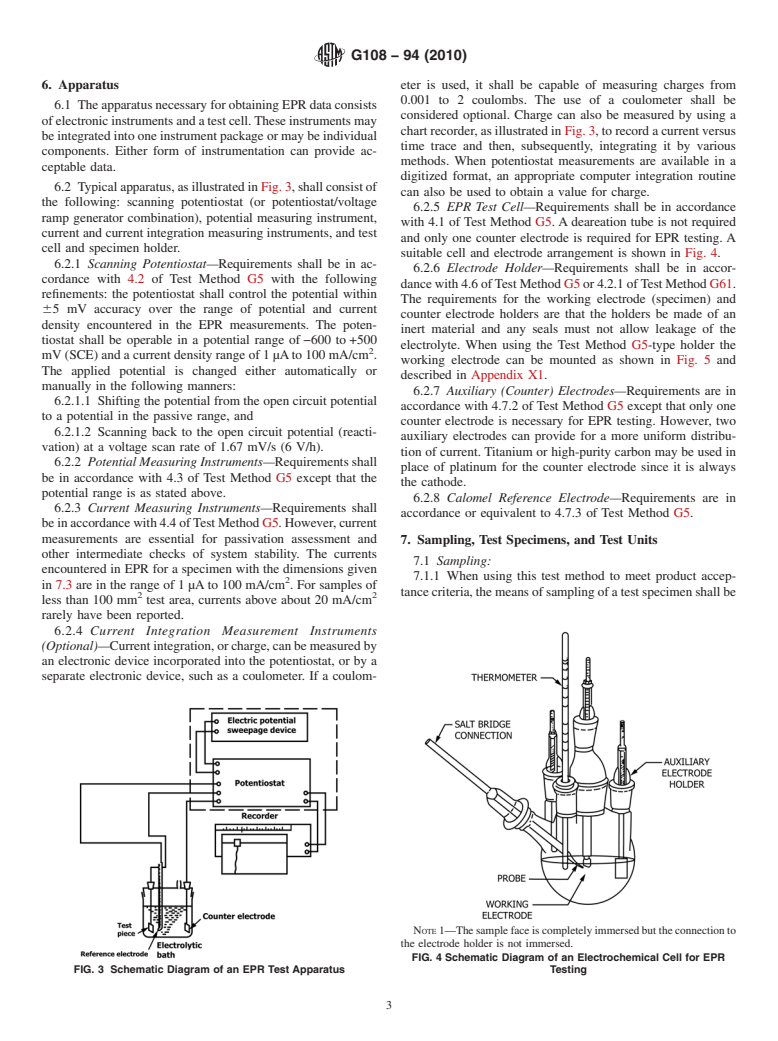
6. Apparatus eter is used, it shall be capable of measuring charges from
0.001 to 2 coulombs. The use of a coulometer shall be
6.1 TheapparatusnecessaryforobtainingEPRdataconsists
considered optional. Charge can also be measured by using a
ofelectronicinstrumentsandatestcell.Theseinstrumentsmay
chartrecorder,asillustratedinFig.3,torecordacurrentversus
beintegratedintooneinstrumentpackageormaybeindividual
time trace and then, subsequently, integrating it by various
components. Either form of instrumentation can provide ac-
methods. When potentiostat measurements are available in a
ceptable data.
digitized format, an appropriate computer integration routine
6.2 Typicalapparatus,asillustratedinFig.3,shallconsistof
can also be used to obtain a value for charge.
the following: scanning potentiostat (or potentiostat/voltage
6.2.5 EPR Test Cell—Requirements shall be in accordance
ramp generator combination), potential measuring instrument,
with 4.1 of Test Method G5.Adeareation tube is not required
current and current integration measuring instruments, and test
and only one counter electrode is required for EPR testing. A
cell and specimen holder.
suitable cell and electrode arrangement is shown in Fig. 4.
6.2.1 Scanning Potentiostat—Requirements shall be in ac-
6.2.6 Electrode Holder—Requirements shall be in accor-
cordance with 4.2 of Test Method G5 with the following
dancewith4.6ofTestMethodG5or4.2.1ofTestMethodG61.
refinements: the potentiostat shall control the potential within
The requirements for the working electrode (specimen) and
65 mV accuracy over the range of potential and current
counter electrode holders are that the holders be made of an
density encountered in the EPR measurements. The poten-
inert material and any seals must not allow leakage of the
tiostat shall be operable in a potential range of−600 to+500
electrolyte. When using the Test Method G5-type holder the
mV(SCE)andacurrentdensityrangeof1µAto100mA/cm .
working electrode can be mounted as shown in Fig. 5 and
The applied potential is changed either automatically or
described in Appendix X1.
manually in the following manners:
6.2.7 Auxiliary (Counter) Electrodes—Requirements are in
6.2.1.1 Shifting the potential from the open circuit potential
accordance with 4.7.2 of Test Method G5 except that only one
to a potential in the passive range, and
counter electrode is necessary for EPR testing. However, two
6.2.1.2 Scanning back to the open circuit potential (reacti-
auxiliary electrodes can provide for a more uniform distribu-
vation) at a voltage scan rate of 1.67 mV/s (6 V/h).
tion of current.Titanium or high-purity carbon may be used in
6.2.2 Potential Measuring Instruments—Requirementsshall
place of platinum for the counter electrode since it is always
be in accordance with 4.3 of Test Method G5 except that the
the cathode.
potential range is as stated above.
6.2.8 Calomel Reference Electrode—Requirements are in
6.2.3 Current Measuring Instruments—Requirements shall
accordance or equivalent to 4.7.3 of Test Method G5.
beinaccordancewith4.4ofTestMethodG5.However,current
measurements are essential for passivation assessment and
7. Sampling, Test Specimens, and Test Units
other intermediate checks of system stability. The currents
7.1 Sampling:
encountered in EPR for a specimen with the dimensions given
7.1.1 When using this test method to meet product accep-
in 7.3 are in the range of 1 µAto 100 mA/cm . For samples of
tancecriteria,themeansofsamplingofatestspecimenshallbe
2 2
less than 100 mm test area, currents above about 20 mA/cm
rarely have been reported.
6.2.4 Current Integration Measurement Instruments
(Optional)—Currentintegration,orcharge,canbemeasuredby
an electronic device incorporated into the potentiostat, or by a
separate electronic device, such as a coulometer. If a coulom-
NOTE1—Thesamplefaceiscompletelyimmersedbuttheconnectionto
the electrode holder is not immersed.
FIG. 4 Schematic Diagram of an Electrochemical Cell for EPR
FIG. 3 Schematic Diagram of an EPR Test Apparatus Testing
G108 − 94 (2010)
7.3.2 Remove any oxides or grease from the specimen as
such film may promote loss of adhesion between the mounting
compoundandthespecimenthatcouldcauseacrevicetoform
thereby producing erroneously high current densities during
the EPR measurement.
7.3.3 The front surface of the specimen will be evaluated in
the EPR test. The back surface of the test specimen is used to
establish electrical contact with the specimen (see Note 2).
NOTE 2—A convenient way to make this attachment may be either by
spot welding or by using a conducting cement to fasten a stainless steel
machinescrew(forexample,NC4-40×0.3cm(0.75in.)long)totheback
surfaceofthespecimen.Thisassemblyismountedinasuitablecompound
that is inert in the EPR electrolyte (see Appendix X1) such that the front
surface upon immersion in the EPR electrolyte is fully in contact with the
electrolyte.
7.3.4 Measurethesurfaceareaofthefrontsurfaceofthetest
specimenwithin0.1mm precisionandrecordontheEPRdata
FIG. 5 A Method of Mounting Specimens for EPR Testing (6) record sheet (see Appendix X2).
7.3.5 Specimens can be in any shape that will not be
susceptible to crevice corrosion in the solution. Test surface
2 2
decided by agreement between the parties
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.