ASTM D3867-99
(Test Method)Standard Test Methods for Nitrite-Nitrate in Water
Standard Test Methods for Nitrite-Nitrate in Water
SCOPE
1.1 These test methods cover the determination of nitrite nitrogen, nitrate nitrogen; and combined nitrite-nitrate nitrogen in water and wastewater in the range from 0.05 to 1.0 mg/L nitrogen. Two test methods are given as follows: Test Method A-Automated Cadmium Reduction (Sections 9-16) and Test Method B-Manual Cadmium Reduction (Sections 17-24).
1.2 These test methods are applicable to surface, saline, waste, and ground waters. It is the user's responsibility to ensure the validity of these test methods for waters of untested matrices.
1.3 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For a specific hazard statement, see 8.2.1.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation: D 3867 – 99
Standard Test Methods for
Nitrite-Nitrate in Water
This standard is issued under the fixed designation D 3867; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope* E 60 Practices for Photometric and Spectrometric Methods
for Chemical Analysis of Metals
1.1 These test methods cover the determination of nitrite
E 275 Practice for Describing and Measuring Performance
nitrogen, nitrate nitrogen, and combined nitrite-nitrate nitrogen
of Ultraviolet, Visible, and Near Infrared Spectrophotom-
in water and wastewater in the range from 0.05 to 1.0 mg/L
eters
nitrogen. Two test methods are given as follows:
Sections
3. Terminology
Test Method A—Automated Cadmium Reduction 9 to 16
Test Method B—Manual Cadmium Reduction 17 to 24
3.1 Definitions: For definitions of terms used in these test
methods, refer to Terminology D 1129.
1.2 These test methods are applicable to surface, saline,
waste, and ground waters. It is the user’s responsibility to
4. Summary of Test Methods
ensure the validity of these test methods for waters of untested
4.1 A filtered sample is passed through a column containing
matrices.
copper-coated cadmium granules to reduce nitrate ion to nitrite
1.3 This standard does not purport to address all of the
ion. The combined nitrite-nitrate nitrogen is determined by
safety concerns, if any, associated with its use. It is the
diazotizing the total nitrite ion with sulfanilamide and coupling
responsibility of the user of this standard to establish appro-
with N-(1-naphthyl)ethylenediamine dihydrochloride to form a
priate safety and health practices and determine the applica-
highly colored azo dye that is measured spectrophotometri-
bility of regulatory limitations prior to use. For specific hazard
cally.
statements, see Note 1 and Note 2.
4.2 The nitrite ion originally present in the sample can be
2. Referenced Documents determined separately by carrying out the procedure and
omitting the cadmium reduction step.
2.1 ASTM Standards:
4.3 The nitrate ion can be calculated as the difference
D 992 Test Method for Nitrate Ion in Water
4 between the combined nitrite-nitrate nitrogen and the nitrite
D 1129 Terminology Relating to Water
nitrogen.
D 1141 Specification for Substitute Ocean Water
D 1192 Specification for Equipment for Sampling Water
5. Significance and Use
and Steam in Closed Conduits
4 5.1 Both test methods use identical reagents and sample
D 1193 Specification for Reagent Water
6 processing. The only difference between the two methods is
D 1254 Test Method for Nitrite Ion in Water
that one test method is automated and the other is manual. The
D 2777 Practice for Determination of Precision and Bias of
4 ranges and interferences are identical.
Applicable Methods of Committee D-19 on Water
5.2 The automated test method is preferred when large
D 3370 Practices for Sampling Water from Closed Con-
4 numbers of samples are to be analyzed. The manual test
duits
method is used for fewer samples or when automated instru-
mentation is not available.
These test methods are under the jurisdiction of ASTM Committee D-19 on
5.3 These test methods replace Test Methods D 1254 (Ni-
Water and are the responsibility of Subcommittee D19.05 on Inorganic Constituents
trite) and D 992 (Nitrate). The nitrite test method (Test Method
in Water.
D 1254) used a reagent which is considered to be a potential
Current edition approved June 10, 1999. Published November 1999. Originally
published as D 3867 – 79. Last previous edition D 3867 – 90. carcinogen. The nitrate test method (Test Method D 992) has
Methods similar to these appear in Methods of Chemical Analysis of Water and
been shown to have relatively large errors when used in
Wastes, 2nd edition, U.S. Environmental Protection Agency.
Discontinued; see 1983 Annual Book of ASTM Standards, Vol 11.01.
Annual Book of ASTM Standards, Vol 11.01.
Annual Book of ASTM Standards, Vol 03.05.
Annual Book of ASTM Standards, Vol 11.02.
6 8
Discontinued; see 1980 Annual Book of ASTM Standards, Part 31. Annual Book of ASTM Standards, Vol 03.06.
*A Summary of Changes section appears at the end of this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 3867 – 99
wastewaters and also has greater manipulative difficulties than 8. Sampling and Sample Preservation
the test method described herein.
8.1 Collect the sample in accordance with Specification
D 1192 and Practices D 3370, as applicable.
6. Interferences
8.2 When nitrite ion is to be determined separately, analyze
6.1 Turbid samples must be filtered prior to analysis to
as soon as possible after sampling. Even when sterile bottles
eliminate particulate interference. Furthermore, sample turbid-
are used, bacteria naturally present in the water may cause
ity results in a buildup on the reduction column that restricts
conversion of all or part of nitrite ion to other forms such as
sample flow.
nitrate or ammonia. Ammonia and natural amines, which are
6.2 Sample color that absorbs at wavelengths between 520
frequently present in natural waters, may react with nitrites to
and 540 nm interferes with the absorbance measurements.
form nitrogen. If samples are to be stored for 24 h or less,
When color is suspect, analyze a sample blank, omitting the
preserve the sample by refrigeration at 4°C. If the sample must
N-(1-naphthyl)ethylenediamine dihydrochloride from the color
be stored for more than 24 h, preserve it by the addition of 2
reagent.
mL of chloroform per litre (11.8 and 11.9) in addition to
6.3 Oil and grease in the sample coat the surface of the
refrigeration at 4°C.
cadmium and prevent complete reduction of nitrate to nitrite.
NOTE 1—WARNING: Chloroform is toxic and is a suspected human
This interference is usually removed by filtration prior to
carcinogen. Use with adequate ventilation or in a fume hood. Wear
analysis. If filtration is not adequate, the interference can be
prescribed protective equipment. Use of chloroform is discouraged, since
removed by preextracting the sample with an n-hexane or a
its use renders the solution a hazardous waste.
solid phase extraction (SPE) filter. NOTE 2—CAUTION: The common prescribed use of sulfuric acid or
mercury compounds as preservatives is discouraged. Sulfuric acid does
6.4 Certain metal ions, in concentrations above 35 mg/L,
not necessarily inhibit oxidation and mercury compounds should be
may cause an interference. For example, Hg (II) and Cu (II)
avoided to prevent environmental pollution. Mercuric chloride is known
may form colored complex ions having absorption bands in the
to deactivate the column.
region of color measurement. Iron and manganese are other
reported examples of interference.
TEST METHOD A—AUTOMATED CADMIUM
6.5 Excessive amounts of chlorine will deactivate the reduc-
REDUCTION
ing column. Chlorine might be present in some Type II water.
9. Scope
The use of chlorine-containing Type II water will lead to a
9.1 The applicable range of this test method is from 0.05 to
negative interference because nitrite and chlorine do not
1 mg/L of nitrite or nitrate nitrogen. The range may be
normally coexist. This is of particular importance when pre-
extended upward by dilution of an appropriate aliquot. Many
paring standards or spiked samples.
workers have found that this test method is reliable for nitrite
6.6 In acid samples (pH less than 4.5) nitrate is not reduced
and combined nitrite-nitrate levels to 0.01 mg N/L. However,
in the cadmium column. To overcome this interference, the
the precision and bias data presented in this test method are
sample must be neutralized to a pH of between 6 and 8 prior to
insufficient to justify application of this test method in the 0.01
analysis.
to 0.05 mg/L-N range.
7. Purity of Reagents 9.2 This test method is applicable to surface, saline, waste,
and ground waters. It is the user’s responsibility to ensure the
7.1 Reagent grade chemicals shall be used in all tests.
validity of this test method for waters of untested matrices.
Unless otherwise indicated, it is intended that all reagents shall
conform to the specifications of the Committee on Analytical
10. Apparatus
Reagents of the American Chemical Society, when such
10.1 Automated Analysis System consisting of:
specifications are available. Other grades may be used, pro-
10.1.1 Sampler.
vided it is first ascertained that the reagent is of sufficient high
10.1.2 Manifold or Analytical Cartridge.
purity to permit its use without lessening the accuracy of the
10.1.3 Colorimeter equipped with a 15- or 50-mm tubular
determination.
flow cell and 540 6 10-nm filters.
7.2 Purity of Water—Unless otherwise indicated, references
10.1.4 Recorder or Electronic Data Acquisition Device.
to water shall be understood to mean reagent water conforming
10.1.5 Digital Printer (Optional).
to Specification D 1193, Type I. Other reagent water types may
10.1.6 Continuous Filter (Optional).
be used, provided it is first ascertained that the water is of
10.2 Reduction Columns—Choose the appropriate reduc-
sufficiently high purity to permit its use without adversely
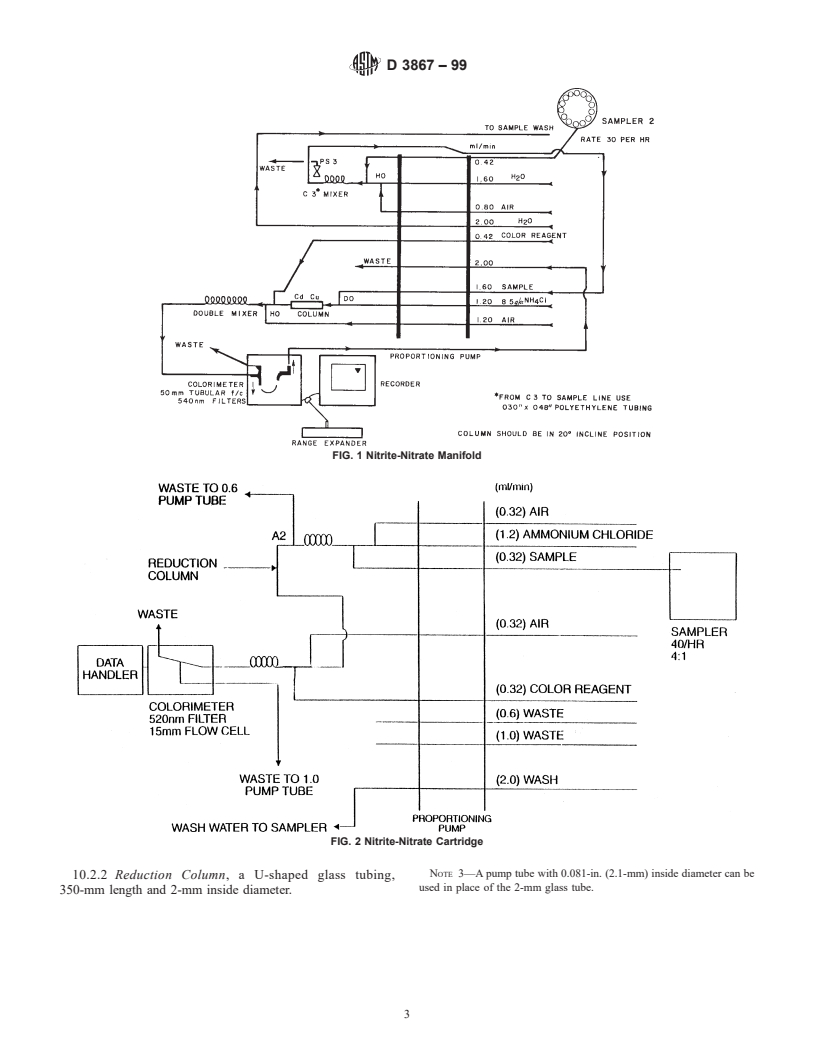
tion column for the manifold system. A schematic drawing of
affecting the bias and precision of these test methods. Type II
the manifold system is shown in Fig. 1 and the cartridge system
water was specified at the time of round-robin testing of these
is shown in Fig. 2.
test methods.
10.2.1 Reduction Column, a glass tube 8 by 50 mm with the
ends reduced in diameter to permit insertion into the system
(see Fig. 1).
“Reagent Chemicals, American Chemical Society Specifications,” American
Chemical Society, Washington, D.C. For suggestions on the testing of reagents not
listed by the American Chemical Society, see Analar Standards for Laboratory The apparatus described is commercially available. ASTM does not undertake
Chemicals, BDH Ltd., Poole, Dorset, U.K. and the United States Pharmacopeia and to ensure anyone utilizing an automated analysis system against liability of
National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, MD. infringement of patent or assume such liability.
D 3867 – 99
FIG. 1 Nitrite-Nitrate Manifold
FIG. 2 Nitrite-Nitrate Cartridge
NOTE 3—A pump tube with 0.081-in. (2.1-mm) inside diameter can be
10.2.2 Reduction Column, a U-shaped glass tubing,
used in place of the 2-mm glass tube.
350-mm length and 2-mm inside diameter.
D 3867 – 99
TABLE 1 Concentration of Calibration Standards, Automated
11. Reagents
Cadmium Reduction
11.1 Ammonium Chloride Solution (85 g/L)—Dissolve 85 g
NO -N or NO -N, mg/L mL Standard Solution/100 mL
3 2
of ammonium chloride (NH Cl) in water and dilute to 1 L. Add
11 0.01 0.1
0.5 mL wetting agent.
0.02 0.2
11.2 Cadmium, 40 to 60 mesh, granulated.
0.04 0.4
0.1 1.0
11.3 Color Reagent— Add the following to 800 mL of
0.2 2.0
water, while stirring constantly: 100 mL of concentrated
0.4 4.0
phosphoric acid (H PO ), 10 g of sulfanilamide, and 0.5 g of
3 4 0.7 7.0
1.0 10.0
N-1-(naphthyl)ethylenediamine dihydrochloride. Stir until dis-
solved. Add 1 mL of wetting agent, and dilute to 1 L with
water. This solution is stable for about a month when stored in
a brown bottle in a dark cool place.
blue color partially fades. Decant and repeat with fresh copper
11.4 Copper Sulfate Solution (20 g/L)—Dissolve 20 g of
sulfate until the first visible brown colloidal precipitate ap-
copper sulfate pentahydrate (CuSO ·5 H O) in 500 mL of
4 2
pears.
water. Dilute to 1 L.
12.1.3 Wash the granules with water at least 10 times to
11.5 n-Hexane.
remove all of the precipitated copper.
11.6 Hydrochloric Acid (1 + 1)—Slowly add 50 mL of
12.2 Filling the Reduction Column:
concentrated hydrochloric acid (HCl) to 40 to 45 mL of water
12.2.1 Insert a small plug of glass wool in one end of the
and dilute to 100 mL.
column (10.2).
11.7 Nitrate Solution, Stock (1.0 mL = 1.0 mg NO -N)—
12.2.2 Fill the column with water to prevent the entrapment
Dry potassium nitrate (KNO ) in an oven at 105°C for 24 h.
of air bubbles during the filling operation.
Dissolve 7.218 g in water in a 1-L volumetric flask. Dilute to
12.2.3 Fill the column with copper-cadmium granules, tap
the mark with water. This solution is stable for up to 1 month
to pack the granules, and plug the open end with glass wool.
with refrigeration. If longer stability is required or refrigeration
12.3 Installation of Reduction Column—Install the copper-
is not available, add 2 mL of chloroform as a preservative and
cadmium reduction column in the automatic analyzer system.
store in a dark bottle. This solution is stable for 6 months. (See
Purge the system with ammonium chloride solution (11.1)
Note 1.)
using water in the sample line. Observe the following precau-
11.8 Nitrate Solution, Standard (1.0 mL = 0.01 mg NO -
tions while installing the reduction column:
N)—Dilute 10 mL of stock nitrate solution (11.7) to 1 L with
12.3.1 Place the column in the manifold system in an
water and store in a dark bottle. Prepare fresh as needed.
upflow 20° incline to minimize channeling (see Fig. 1).
11.9 Nitrite Solution, Stock (1.0 mL = 1.0 mg NO -N)—
12.3.2 Fill all pump tubes with reagents before inserting the
Place about7gof potassium nitrite (KNO ) in a tared 125-mL
column in the cartridge system to prevent the entrapment of air
beaker and dry for about 24 h to a constant weight in a
bubbles.
desiccator containing a suitable dessicant. Adjust the weight of
12.4 Reduction Column Storage—When it is not in use, put
the dry potassium nitrite to 6.072 g. Add 50 mL of water to the
the sample line in water and purge the column with ammonium
beaker, stir until dissolved, and transfer quantitatively to a
chloride solution and water.
1000-mL volumetric flask. Dilute to the mark with water store
NOTE 5—Do not allow air to enter the column and do not let the
in a sterilized bottle under refrigeration. Prepare fresh as
cadmium granules become dry. If this occurs, refill the column with
needed.
freshly treated cadmium granules.
NOTE 4—Potassium nitrite is easily oxidized, so use only fresh bottles
13. Calibration
of this reagent.
13.1 Using the standard nitrate solution (11.8) prepare
11.10 Nitrite Solution, Standard (1.0 mL = 0.01 mg NO -
calibration standards by pipetting specified volumes of the
N)—Dilute 10 mL of stock nitrite solution (11.9) to 1 L with
s
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.