ASTM D4251-89(2009)
(Test Method)Standard Test Method for Active Matter in Anionic Surfactants by Potentiometric Titration
Standard Test Method for Active Matter in Anionic Surfactants by Potentiometric Titration
SIGNIFICANCE AND USE
Anionic surfactants are the most widely used of the synthetic detergents. ASTM methods in current use for their determination involve two-phase aqueous/chloroform titrations with the organic dyes methylene blue (Test Method D1681) or disulphine blue/dimidium bromide (Test Method D3049) as indicators. One advantage of the potentiometric method is that it eliminates the use of chloroform whose use is restricted for environmental and toxicological reasons.
This test method is intended for use as described in 1.1.
SCOPE
1.1 This test method describes a potentiometric titration procedure for determining the anionic active matter in detergents. It is intended for the analysis of anionic surfactants such as detergent range alkylbenzenesulfonates, α-olefin sulfonates, alcohol sulfates, and alcohol ethosulfates. It has not been tested for surfactant formulations.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Material Safety Data Sheets are available for reagents and materials. Review them for hazards prior to usage.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D4251 − 89(Reapproved 2009)
Standard Test Method for
Active Matter in Anionic Surfactants by Potentiometric
Titration
This standard is issued under the fixed designation D4251; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Terminology
3.1 Definitions of Terms Specific to This Standard:
1.1 This test method describes a potentiometric titration
3.1.1 active matter—the organic surface-active material
procedure for determining the anionic active matter in deter-
present in the detergent and defined in Terminology D459 as
gents.Itisintendedfortheanalysisofanionicsurfactantssuch
active ingredient of a synthetic detergent.
as detergent range alkylbenzenesulfonates, α-olefin sulfonates,
alcoholsulfates,andalcoholethosulfates.Ithasnotbeentested
4. Summary of Test Method
for surfactant formulations.
4.1 A detergent sample containing active matter is titrated
1.2 The values stated in SI units are to be regarded as
potentiometrically in an aqueous medium with a standard
standard. No other units of measurement are included in this
solution of Hyamine 1622 using a nitrate ion-selective elec-
standard.
trode. The titration reaction involves the formation of a
1.3 This standard does not purport to address all of the complex between the cationic quaternary ammonium titrant
safety concerns, if any, associated with its use. It is the
(Hyamine 1622) and the anionic surfactant which precipitates.
responsibility of the user of this standard to establish appro- Thenitrateelectrodeprobablyrespondstotheconcentrationof
priate safety and health practices and determine the applica-
unreacted anionic surfactant.
bility of regulatory limitations prior to use. Material Safety
5. Significance and Use
Data Sheets are available for reagents and materials. Review
them for hazards prior to usage.
5.1 Anionic surfactants are the most widely used of the
synthetic detergents. ASTM methods in current use for their
2. Referenced Documents
determinationinvolvetwo-phaseaqueous/chloroformtitrations
2 with the organic dyes methylene blue (Test Method D1681)or
2.1 ASTM Standards:
disulphine blue/dimidium bromide (Test Method D3049)as
D459Terminology Relating to Soaps and Other Detergents
indicators. One advantage of the potentiometric method is that
D1193Specification for Reagent Water
it eliminates the use of chloroform whose use is restricted for
D1681Test Method for SyntheticAnionicActive Ingredient
environmental and toxicological reasons.
in Detergents by Cationic Titration Procedure
5.2 This test method is intended for use as described in 1.1.
D3049Test Method for Synthetic Anionic Ingredient by
Cationic Titration
6. Apparatus
E180Practice for Determining the Precision of ASTM
Methods for Analysis and Testing of Industrial and Spe-
6.1 Potentiometric Titration Assembly, consisting of an
cialty Chemicals (Withdrawn 2009)
automatic titrator (Metrohm E536 or equivalent) fitted with a
nitrateion-selectiveelectrode(OrionModel93-07NitrateISE,
or equivalent) and a Ag/AgCl reference electrode (Metrohm
EA440 or equivalent) together with a buret assembly having a
This test method is under the jurisdiction ofASTM Committee D12 on Soaps
5-mL buret (Metrohm E575) and 150-mL beaker. A TFE-
and Other Detergents and is the direct responsibility of Subcommittee D12.12 on
AnalysisandSpecificationsofSoaps,Synthetics,DetergentsandtheirComponents.
fluorocarbon star-head stirring bar can be used to provide
Current edition approved Oct. 1, 2009. Published December 2009. Originally
mixing and eliminate foaming during titration. Use of the
approved in 1983. Last previous edition approved in 2003 as D4251–89(2003).
Orion electrode with a Metrohm E536 requires an adapter
DOI: 10.1520/D4251-89R09.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or (Metrohm EA-1046/2).
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
NOTE 1—The conditioning of the electrode is essential for obtaining a
Standards volume information, refer to the standard’s Document Summary page on
good break in the titration curve. Conditioning new electrodes in 0.01 M
the ASTM website.
The last approved version of this historical standard is referenced on NaNO aqueous solution for 60 min (or more) prior to use is recom-
www.astm.org. mended. Condition previously used electrodes by using again for the
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D4251 − 89 (2009)
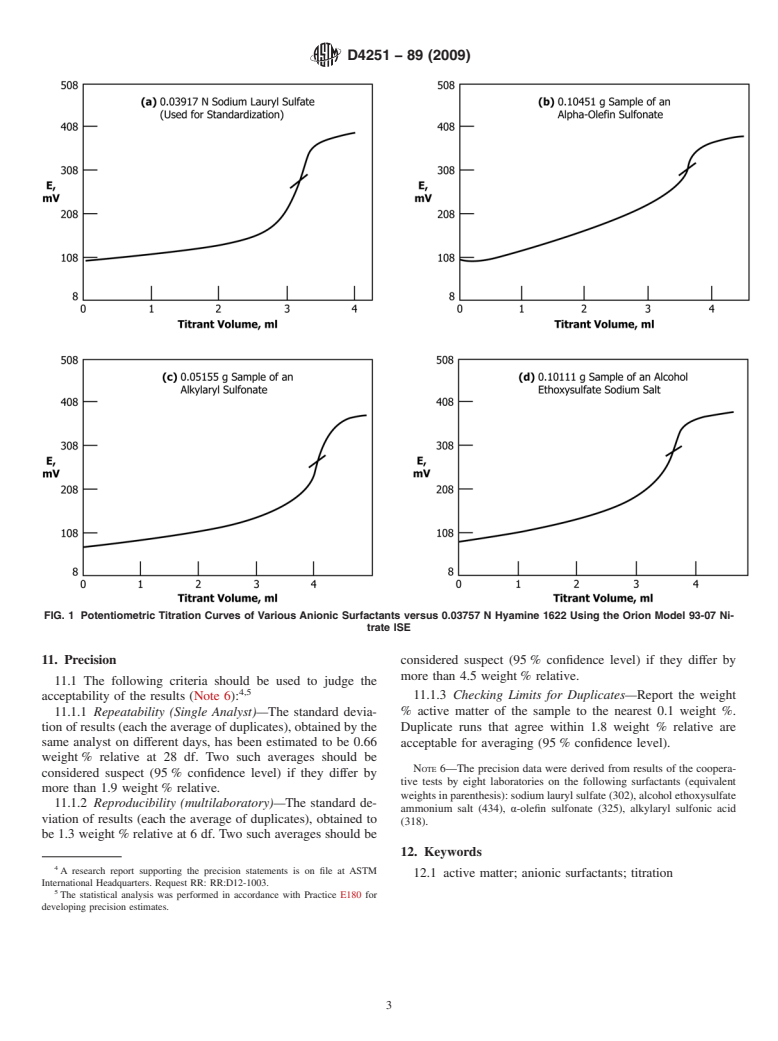
TABLE 1 Active Matter Content of Various Anionic Surfactants by
B = normality of sodium lauryl sulfate, and
the Potentiometric Titration and Two-Phase Titration Methods
C = Hyamine solution consumed during titration, mL.
Active Matter Content, % weight
Potentiometric Titration Two-
Anionic Surfactant
9. Procedure
Orion Orion “Old” HNU Phase
− − −
NO (#1) NO (#2) NO Titration
3 3 3
A 9.1 Add to a 150-mL beaker a known weight of detergent
Sulframin AOS (alpha- 41.03 41.49 40.91 39.21
39.26
olefin sulfonate) 42.50 40.74 41.05
sample together with enough water to make 50 mLof solution
42.64 41.34 41.05
(Note 4). The solution should cover the sensing tips of the
B
Sulframin 1298 (alkylaryl 94.15 96.26 94.91 95.12
95.12 electrodes. Titrate initially by adding Hyamine solution at
sulfonate) 95.31 95.67 95.50
94.73 96.26 94.91
approximately 0.5 mL/min while stirring constantly. As the
B
NEODOL® 25-3S (alcohol 58.12 57.94 57.75 58.19
inflection point is approached, reduce the addition rate, and
58.24
ethoxysulfate sodium 58.81 57.74 57.95
continue titrating well past the inflection in the titration curve.
salt) 58.12 57.94 57.95
A
(Automatic titrators can be preset to automatically slow down
Methylene blue method.
B
Mixed indicator method. the addition rate as the inflection point is approached.)
NOTE 4—To determine the amount of sample needed for an approxi-
mate 3.75-mL titration (0.15 meq) use the following equation:
titration of aqueous sodium lauryl sulfate with Hyamine.
~0.015!M
NOTE 2—Other electrodes (for ex
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.