ASTM D2766-95
(Test Method)Standard Test Method for Specific Heat of Liquids and Solids
Standard Test Method for Specific Heat of Liquids and Solids
SCOPE
1.1 This test method covers the determination of the heat capacity of liquids and solids. It is applicable to liquids and solids that are chemically compatible with stainless steel, that have a vapor pressure less than 13.3 kPa (100 torr), and that do not undergo phase transformation throughout the range of test temperatures. The specific heat of materials with higher vapor pressures can be determined if their vapor pressures are known throughout the range of test temperatures.
1.2 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: D 2766 – 95 An American National Standard
Standard Test Method for
Specific Heat of Liquids and Solids
This standard is issued under the fixed designation D 2766; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope
R 5 resistance of nominal 100-V standard resistor,
R 5 resistance of nominal 10 000-V standard re-
1.1 This test method covers the determination of the heat 10 000
sistor,
capacity of liquids and solids. It is applicable to liquids and
E 5 emf across nominal 1-V standard resistor,
solids that are chemically compatible with stainless steel, that
E 5 emf across nominal 100-V standard resistor,
have a vapor pressure less than 13.3 kPa (100 torr), and that do
E 5 emf across nominal 10 000-V standard resis-
10 000
not undergo phase transformation throughout the range of test
tor,
temperatures. The specific heat of materials with higher vapor
t 5 time of application of calibration heater cur-
c
pressures can be determined if their vapor pressures are known
rent, s,
throughout the range of test temperatures.
q 5 total heat developed by calibration heater, cal,
1.2 The values stated in SI units are to be regarded as the
DE 5 total heat effect for container, mV,
c
standard. The values given in parentheses are for information
DE 5 total heat effect for sample + container, mV,
s
only.
De 5 total heat effect for calibration of calorimeter
c
1.3 This standard does not purport to address all of the
system during container run, mV,
safety concerns, if any, associated with its use. It is the
De 5 total heat effect for calibration of calorimeter
s
responsibility of the user of this standard to establish appro-
system during sample run, mV,
priate safety and health practices and determine the applica-
DH 5 total enthalpy change for container changing
c
bility of regulatory limitations prior to use.
from T to T ,
f c
DH 5 total enthalpy change for sample plus con-
T
2. Referenced Documents
tainer changing from T to T ,
f c
2.1 ASTM Standards:
DH 5 total enthalpy change for sample changing
s
D 1217 Test Method for Density and Relative Density
from T to T ,
f c
(Specific Gravity) of Liquids by Bingham Pycnometer F 5 calorimeter factor,
W 5 weight of sample corrected for air buoyancy
3. Terminology
d 5 density of sample at T ,
f f
d 5 density of sample at T ,
3.1 Definitions of Terms Specific to This Standard:
c c
V 5 total volume of sample container,
3.1.1 specific heat—the ratio of the amount of heat needed T
V 5 volume of sample vapor at T ,
to raise the temperature of a mass of the substance by a f f
V 5 volume of sample vapor at T ,
c c
specified amount to that required to raise the temperature of an
P 5 vapor pressure of sample at T ,
f f
equal mass of water by the same amount, assuming no phase
P 5 vapor pressure of sample at T ,
c c
change in either case.
N 5 moles sample vapor at T ,
f f
3.2 Symbols:Symbols:
N 5 moles sample vapor at T ,
c c
N 5 moles sample vapor condensed,
DH 5 heat of vaporization of sample,
v
T 5 temperature of hot zone, °C,
f
R 5 gas constant, and
T 5 initial temperature of calorimeter, °C,
c
K 5 heat of vaporization correction.
T8 5 T −T 5 temperature differential, °C,
f c
3.3 Units:
R 5 resistance of nominal 1-V standard resistor,
3.3.1 The energy and thermal (heat) capacity units used in
this method are defined as follows:
1 cal (International Table) 5 4.1868 J
This test method is under jurisdiction of ASTM Committee D-2 on Petroleum 1 Btu (British thermal unit, International Table) 5
Products and Lubricants and is the direct responsibility of Subcommittee D02.11 on
1055.06 J
Engineering Science of High Performance Fluids and Solids.
1 Btu/lb °F 5 1 cal/g °C
Current edition approved Aug. 15, 1995. Published October 1995. Originally
1 Btu/lb °F 5 4.1868 J/g K
published as D 2766 – 68 T. Last previous edition D 2766 – 91.
Annual Book of ASTM Standards, Vol 05.01.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
D 2766
3.3.2 For all but the most precise measurements made with
this method the rounded-off value of 4.19 J/cal can be used as
this is adequate for the precision of the test and avoids the
difficulty caused by the dual definition of the calorie.
4. Summary of Test Method
4.1 The enthalpy change, DH , that occurs when an empty
c
sample container is transferred from a hot zone of constant
temperature to an adiabatic calorimeter at a fixed initial
temperature is measured for selected hot zone temperatures
evenly spread over the temperature range of interest.
4.2 The enthalpy change, DH , that occurs when a container
T
filled with the test specimen is transferred from a hot zone of
constant temperature, T , to an adiabatic calorimeter at a fixed
c
initial temperature is measured for selected hot-zone tempera-
tures evenly spread over the temperature range of interest.
4.3 The net enthalpy change per gram of sample is then
expressed as an analytical power function of the temperature
differential T8. The first derivative of this function with respect
to the actual temperature, T , yields the specific heat of the
f
sample as a function of temperature. Actual values of the
specific heat may be obtained from solutions of this equation
which is valid over the same range of temperatures over which
the total enthalpy changes, DH , were measured.
T
5. Significance and Use
5.1 The specific heat or heat capacity of a substance is a
thermodynamic property that is a measure of the amount of
energy required to produce a given temperature change within
a unit quantity of that substance. It is used in engineering
calculations that relate to the manner in which a given system
may react to thermal stresses.
6. Apparatus
6.1 Drop-Method-of-Mixtures Calorimeter, consisting es-
sentially of a vertically mounted, thermostatically controlled,
FIG. 1 Specific Heat Apparatus
tube furnace and a water-filled adiabatic calorimeter. The
furnace is mounted with respect to the calorimeter in such a
6.4 Resistor,1-V precision type.
way that it may be swung from a remote position to a location
6.5 Resistor, 100-V precision type.
directly over the calorimeter and returned rapidly to the remote
6.6 Resistor, 10 000-V precision type.
position. The sample container may thus be dropped directly
6.7 Amplifier, zero centered range, linear response with
into the calorimeter with a minimum transfer of radiation from
preset ranges to include 625 μnV,6 100 μV, 6200 μV, 6500μ
furnace to calorimeter. Details of construction are shown in
V, 61000 μV, and 62000 μV; with error not to exceed
Fig. 1.
60.04 % of output; with zero drift after warm-up not to exceed
6.2 Sample Container—A stainless steel sample container
60.5 μV offset within which drift will not exceed 60.2
with a polytetrafluoroethylene seal suitable for use at tempera-
μV/min. Equivalent instrumentation with different fixed poten-
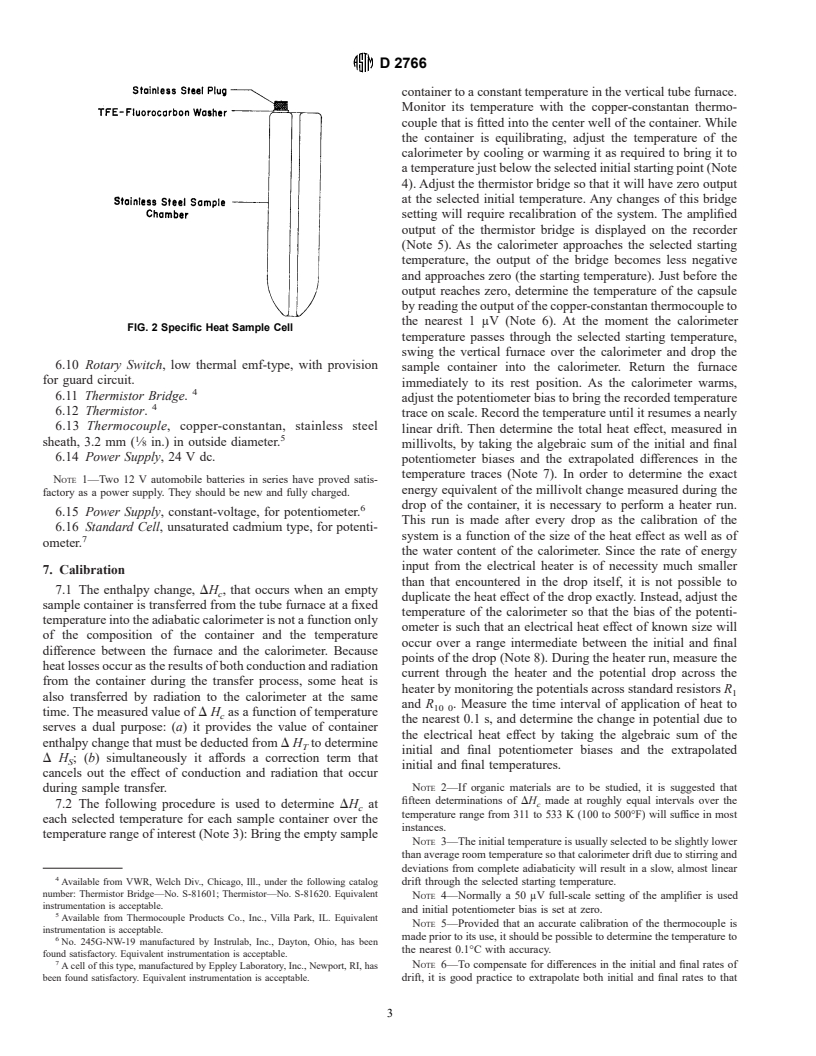
tures up to 533 K (500°F) is shown in Fig. 2.
tial ranges is acceptable provided the same overall potential
6.3 Potential Measuring Devices (two required), potential ranges are covered.
measuring device capable of measurement of up to 1 V with a 6.8 Strip Chart Recorder, with nominal 25 cm chart, 65
−6
mV, zero center.
precision of 10 V or a potentiometer assembly with sensitiv-
ity of at least 1 μV or a digital multimeter with equivalent 6.9 Binding Posts, low thermal emf-type, with provision for
guard circuit.
sensitivity, range, and a minimum of six digit resolution is
acceptable. A direct reading digital temperature indicating
device may be substituted for the potential measuring device
Models 9330/1, 9330/100, 9330/10K manufactured by Guidlines Instruments,
for the purpose of measuring the temperature of the capsule
Inc., 103 Commerce St., Ste 160, Lake May, FL 32795-2590. Equivalent instru-
while in the tube furnace. See Fig. 3. mentation is acceptable.
D 2766
container to a constant temperature in the vertical tube furnace.
Monitor its temperature with the copper-constantan thermo-
couple that is fitted into the center well of the container. While
the container is equilibrating, adjust the temperature of the
calorimeter by cooling or warming it as required to bring it to
a temperature just below the selected initial starting point (Note
4). Adjust the thermistor bridge so that it will have zero output
at the selected initial temperature. Any changes of this bridge
setting will require recalibration of the system. The amplified
output of the thermistor bridge is displayed on the recorder
(Note 5). As the calorimeter approaches the selected starting
temperature, the output of the bridge becomes less negative
and approaches zero (the starting temperature). Just before the
output reaches zero, determine the temperature of the capsule
by reading the output of the copper-constantan thermocouple to
the nearest 1 μV (Note 6). At the moment the calorimeter
FIG. 2 Specific Heat Sample Cell
temperature passes through the selected starting temperature,
swing the vertical furnace over the calorimeter and drop the
6.10 Rotary Switch, low thermal emf-type, with provision
sample container into the calorimeter. Return the furnace
for guard circuit.
immediately to its rest position. As the calorimeter warms,
6.11 Thermistor Bridge.
adjust the potentiometer bias to bring the recorded temperature
6.12 Thermistor.
trace on scale. Record the temperature until it resumes a nearly
6.13 Thermocouple, copper-constantan, stainless steel
linear drift. Then determine the total heat effect, measured in
sheath, 3.2 mm ( ⁄8 in.) in outside diameter.
millivolts, by taking the algebraic sum of the initial and final
6.14 Power Supply, 24 V dc.
potentiometer biases and the extrapolated differences in the
temperature traces (Note 7). In order to determine the exact
NOTE 1—Two 12 V automobile batteries in series have proved satis-
energy equivalent of the millivolt change measured during the
factory as a power supply. They should be new and fully charged.
drop of the container, it is necessary to perform a heater run.
6.15 Power Supply, constant-voltage, for potentiometer.
This run is made after every drop as the calibration of the
6.16 Standard Cell, unsaturated cadmium type, for potenti-
system is a function of the size of the heat effect as well as of
ometer.
the water content of the calorimeter. Since the rate of energy
input from the electrical heater is of necessity much smaller
7. Calibration
than that encountered in the drop itself, it is not possible to
7.1 The enthalpy change, DH , that occurs when an empty
c
duplicate the heat effect of the drop exactly. Instead, adjust the
sample container is transferred from the tube furnace at a fixed
temperature of the calorimeter so that the bias of the potenti-
temperature into the adiabatic calorimeter is not a function only
ometer is such that an electrical heat effect of known size will
of the composition of the container and the temperature
occur over a range intermediate between the initial and final
difference between the furnace and the calorimeter. Because
points of the drop (Note 8). During the heater run, measure the
heat losses occur as the results of both conduction and radiation
current through the heater and the potential drop across the
from the container during the transfer process, some heat is
heater by monitoring the potentials across standard resistors R
also transferred by radiation to the calorimeter at the same
and R . Measure the time interval of application of heat to
10 0
time. The measured value of D H as a function of temperature
c
the nearest 0.1 s, and determine the change in potential due to
serves a dual purpose: (a) it provides the value of container
the electrical heat effect by taking the algebraic sum of the
enthalpy change that must be deducted from D H to determine
T
initial and final potentiometer biases and the extrapolated
D H;(b) simultaneously it affords a correction term that
S
initial and final temperatures.
cancels out the effect of conduction and radiation that occur
NOTE 2—If organic materials are to be studied, it is suggested that
during sample transfer.
fifteen determinations of DH made at roughly equal intervals over the
7.2 The following procedure is used to determine DH at c
c
temperature range from 311 to 533 K (100 to 500°F) will suffice in most
each selected temperature for each sample container over the
instances.
temperature range of interest (Note 3): Bring the empty sample
NOTE 3—The initial temperature is usually selected to be slightly lower
than average room temperature so that calorimeter drift due to stirring and
deviations from complete adiabaticity will result in a slow, almost linear
Available from VWR, Welch Div., Chicago, Ill., under the following catalog drift through the selected starting temperature.
number: Thermistor Bridge—No. S-81601; Thermistor—No. S-81620. Equivalent
NOTE 4—Normally a 50 μV full-scale setting of the amplifier is used
instrumentation is acceptable.
and initial potentiometer bias is set at zero.
Available from Thermocouple Products Co., Inc., Villa Park, IL. Equivalent
NOTE 5—Provided that an accurate calibration of the thermocouple is
instrumentation is acceptable.
6 made prior to its use, it should be possible to determine the temperature to
No. 245G-NW-19 manufactured by Instrulab, Inc., Dayton, Ohio, has been
the nearest 0.1°C with accuracy.
found satisfactory. Equivalent instrumentation is acceptable.
NOTE 6—To compensate for differences in the initial and final rates of
A cell of this type, manufactured by Eppley Laboratory, Inc., Newport, RI, has
been found satisfactory. Equivalent instrumentation is acceptable. drift, it is good practice to extrapolate both initial and final rates to that
D 2766
FIG. 3 Specific Heat Measuring and Control Circuit Diagram
point in time at which one half of the total heat effect has occurred. For the
in the Preparation of Apparatus section of Method D 1217.
heat effect occurring after a drop, it has been found that one half of the
Repeat the procedure described in 7.2 for each temperature at
total heat effect occurs so rapidly that no significant error occurs in
which it is desired to determine the value of DH for the filled
T
extrapolating the final drift back to the initial time. For heater runs, it is
sample container. The number of determinations neede
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.