ASTM E2475-10(2016)
(Guide)Standard Guide for Process Understanding Related to Pharmaceutical Manufacture and Control
Standard Guide for Process Understanding Related to Pharmaceutical Manufacture and Control
SCOPE
1.1 The purpose of this guide is to establish a framework and context for process understanding for pharmaceutical manufacturing using quality by design (QbD) (Juran, 1992;2 FDA/ICH Q8). The framework is applicable to both active pharmaceutical ingredient (API) and to drug product (DP) manufacturing. High (detailed) level process understanding can be used to facilitate production of product which consistently meets required specifications. It can also play a key role in continuous process improvement efforts.
1.2 Process Analytical Technology (PAT) is one element that can be used for achieving control over those inputs determined to be critical to a process. It is important for the reader to recognize that PAT is defined as:
“…a system for designing, analyzing, and controlling manufacturing through timely measurements (i.e., during processing) of critical quality and performance attributes of raw and in process materials and processes, with the goal of ensuring final product quality. It is important to note that the term analytical in PAT is viewed broadly to include chemical, physical, microbiological, mathematical, and risk analysis conducted in an integrated manner. The goal of PAT is to enhance understanding and control the manufacturing process…” (U.S. FDA PAT)
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E2475 − 10 (Reapproved 2016)
Standard Guide for
Process Understanding Related to Pharmaceutical
Manufacture and Control
This standard is issued under the fixed designation E2475; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope E2474 Practice for Pharmaceutical Process Design Utilizing
Process Analytical Technology (Withdrawn 2020)
1.1 The purpose of this guide is to establish a framework
E2617 Practice for Validation of Empirically Derived Mul-
and context for process understanding for pharmaceutical
2 tivariate Calibrations
manufacturing using quality by design (QbD) (Juran, 1992;
2.2 U.S. Government Publications:
FDA/ICH Q8). The framework is applicable to both active
FDA/ICH Q8 Pharmaceutical Development
pharmaceutical ingredient (API) and to drug product (DP)
FDA/ICH Q10 Pharmaceutical Quality Systems
manufacturing. High (detailed) level process understanding
U.S. FDA PAT Guidance Document, Guidance for Industry
can be used to facilitate production of product which consis-
PAT—A Framework for Innovative Pharmaceutical
tently meets required specifications. It can also play a key role
Manufacturing and Quality Assurance
in continuous process improvement efforts.
3. Terminology
1.2 Process Analytical Technology (PAT) is one element
that can be used for achieving control over those inputs
3.1 Definitions of Terms Specific to This Standard:
determined to be critical to a process. It is important for the
3.1.1 critical inputs, n—critical process parameters and
reader to recognize that PAT is defined as:
critical raw material attributes for a given process.
“{a system for designing, analyzing, and controlling manufacturing through
American Society for Quality
timely measurements (i.e., during processing) of critical quality and performance
3.1.2 empirical, adj—any conclusion based on experimental
attributes of raw and in process materials and processes, with the goal of
ensuring final product quality. It is important to note that the term analytical in
data and past experience, rather than on theory.
PAT is viewed broadly to include chemical, physical, microbiological,
3.1.3 expert system, n—an expert system is a computer
mathematical, and risk analysis conducted in an integrated manner. The goal of
PAT is to enhance understanding and control the manufacturing process{”
program that simulates the judgment and behavior of a human
(U.S. FDA PAT)
or an organization that has expert knowledge and experience in
1.3 This standard does not purport to address all of the
a particular field.
safety concerns, if any, associated with its use. It is the
3.1.3.1 Discussion—Typically, such a system contains a
responsibility of the user of this standard to establish appro-
knowledge base containing accumulated experience and a set
priate safety and health practices and determine the applica-
of rules for applying the knowledge base to each particular
bility of regulatory limitations prior to use.
situation that is described to the program. Sophisticated expert
systems can be enhanced with additions to the knowledge base
2. Referenced Documents
or to the set of rules.
2.1 ASTM Standards:
3.1.4 first principles, n—a calculation is said to be from first
E456 Terminology Relating to Quality and Statistics
principles, or ab initio, if it starts directly at the level of
E2281 Practice for Process Capability and Performance
established laws of physics and does not make assumptions
Measurement
such as model and fitting parameters.
3.1.5 mechanistic, adj—(1) of, or relating to, theories that
This guide is under the jurisdiction of ASTM Committee E55 on Manufacture
explain phenomena in purely physical or deterministic terms: a
of Pharmaceutical and Biopharmaceutical Products and is the direct responsibility of
Subcommittee E55.11 on Process Design.
mechanistic interpretation of nature.
Current edition approved Sept. 1, 2016. Published September 2016. Originally
approved in 2010. Last previous edition approved in 2010 as E2475 – 10.
DOI:10.1520/E2475-10R16. The last approved version of this historical standard is referenced on
Juran, J., Juran on Quality by Design: The New Steps for Planning Quality Into www.astm.org.
Goods and Services, Free Press, New York, N.Y., 1992. Available from U.S. Government Printing Office Superintendent of Documents,
For referenced ASTM standards, visit the ASTM website, www.astm.org, or 732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401, http://
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM www.access.gpo.gov.
Standards volume information, refer to the standard’s Document Summary page on Available from American Society for Quality (ASQ), 600 N. Plankinton Ave.,
the ASTM website. Milwaukee, WI 53203, http://www.asq.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2475 − 10 (2016)
3.1.6 process capability, n—statistical estimate of the out- 4.3.2 Accordingly, the development of process understand-
come of a characteristic from a process that has been demon- ing should be treated as an ongoing process. Learning should
strated to be in a state of statistical control. E2281 continue throughout the product and process life cycle to
improve the level of process understanding to include process
3.1.7 process inputs, n—the combination of all process
parameters and other factors (for example, environmental,
parameters and raw material attributes for a given process.
changes of scale, changes in raw materials, changes in person-
3.1.8 process understanding, v—to recall and comprehend
nel) which may have changed or which may have newly
process knowledge such that product quality can be explained
emerged since the time the process was first commissioned.
logically or scientifically, or both, as a function of process
Work to enhance process understanding continuously through-
inputs and respond accordingly.
out the life cycle of the product and process can provide
assurance that the process will continue to have an acceptably
3.1.9 residual error, n—the difference between the observed
result and the predicted value (estimated treatment response); low risk of producing out of specification results.
4.3.3 Manufacturers are encouraged to continuously moni-
Observed Result minus Predicted Value. E456
tor and improve upon their operations to enhance product
3.1.10 uncertainty, n—an indication of the variability asso-
quality.
ciated with a measured value that takes into account two major
components of error: (1) bias, and (2) the random error 4.4 Process Understanding for the Whole Process:
attributed to the imprecision of the measurement process. E456 4.4.1 For each product, process understanding covers the
process from the initial design of the chemical or biological
drug substance through manufacturing of the unit dose or
4. Process Understanding
device to final packaging. In addition, the critical quality
4.1 From physical, chemical, biological, and microbiologi-
attributes of the raw materials will in turn become inputs to the
cal perspectives, a process is considered to be well understood
drug product manufacturing process, as will process param-
when:
eters.
(1) All significant sources of variability in process inputs
4.4.2 Fig. 1 schematically illustrates that the performance of
are identified and explained,
any process output (Y) is a function of the inputs (X), which can
(2) The effect of these sources of variability on product
be classified into one of six categories (that is, operator,
quality attributes can be accurately and reliably estimated
equipment, measurements, methods, materials, and environ-
based on the inputs to the process, and
mental conditions).
(3) Significant process parameters are continuously man-
4.4.3 Comprehensive understanding of the relationships of
aged and controlled to ensure that the process must produce
the process inputs and operating parameters to quality attri-
product which is continuously within required specifications to
butes of the resulting product is fundamental to developing a
the user specified required degree or confidence.
successful risk mitigation or control strategy, or both. Identi-
4.2 A well-controlled process is a process where the risk of
fication of critical process parameters (CPPs) and critical raw
producing product not meeting required specifications is below
material attributes should be carried out using suitable experi-
the maximum acceptable level of risk as predetermined by the
mental and investigative techniques. An understanding of these
user. Accordingly, process understanding requires the compre-
critical inputs (CPPs and critical raw material attributes), and
hension and recall of process knowledge sufficient for the
their monitoring and control, is essential when designing a
logical, statistical, or scientific understanding, or combination
process that is able to consistently and reliably deliver product
thereof, of how significant process parameters and raw material
of the desired quality.
attributes relate to, or impact the quality attributes of, the
4.4.4 One method for achieving the desired state is through
product being produced. Sufficient process understanding
multivariate analysis and control. The acceptable operating
should be achieved to reduce risk to an acceptable level for the
envelope of the critical inputs defines the relationship between
patient, manufacturer, or any other stakeholder.
the design space, control strategy and operating range(s).
4.4.5 Note that for raw materials, an additional source of
4.3 A Lifecycle Commitment (Development and Commercial
variability derives from the potential for adulteration. This
Manufacture):
requires that manufacturers understand their incoming supply
4.3.1 Process understanding is fundamental to QbD. It is
chain and suppliers quality systems, and include methods to
important to realize that due to commercial realities (for
detect adulteration of materials in addition to confirming
example, finite resources, time, and money), a process will
identity as necessary, bearing in mind that adulteration may be
typically be commissioned as soon as the degree of process
difficult to detect by standard methods. It also requires that
understanding is sufficient to permit operation of the process
manufacturers use suppliers that are aware of these concerns
with an acceptably low, user specified, level of risk of
and are prepared to implement their own precautionary
producing out of specification product. While it may be
measures, and to permit transparency into their respective
appropriate to commission a process once this minimum
supply sources.
degree of process understanding is achieved, the risk that the
4.5 Tools of Process Understanding:
process may transition out of control steadily increases over
time (for example, process drift), and could exceed the 4.5.1 Process understanding begins with process design
maximum acceptable risk without warning, unless an ongoing (Practice E2474) and usually a structured, small scale devel-
program to enhance process understanding is in place. opment program which focuses on efficiently delivering a
E2475 − 10 (2016)
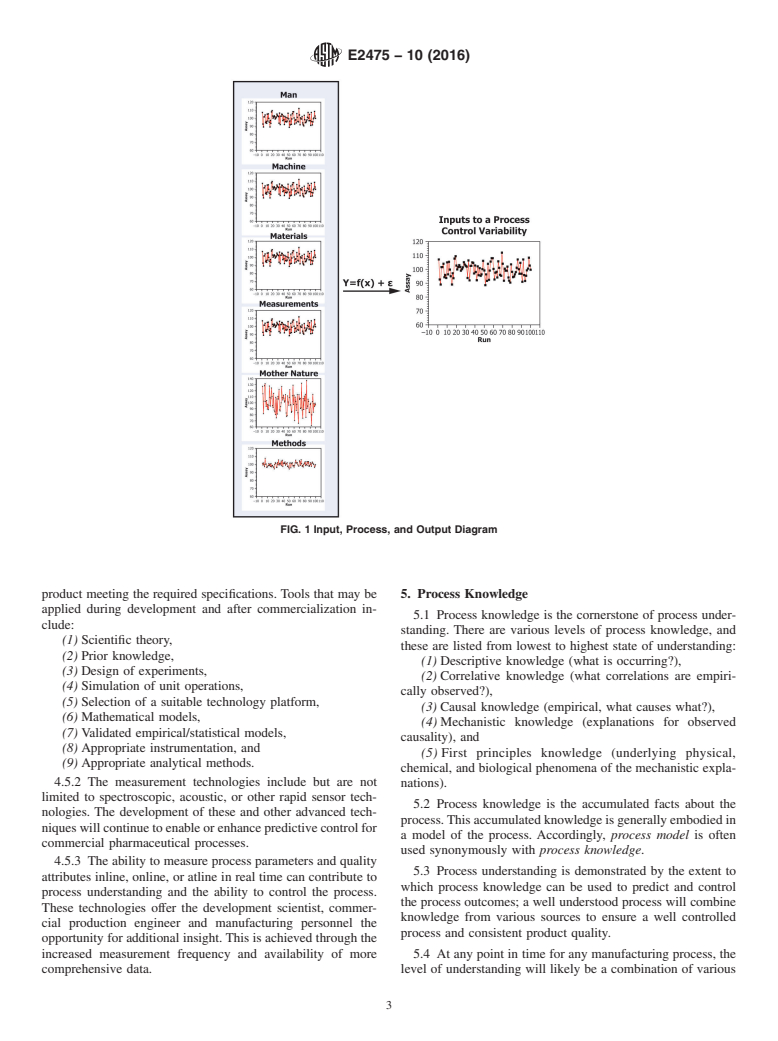
FIG. 1 Input, Process, and Output Diagram
product meeting the required specifications. Tools that may be 5. Process Knowledge
applied during development and after commercialization in-
5.1 Process knowledge is the cornerstone of process under-
clude:
standing. There are various levels of process knowledge, and
(1) Scientific theory,
these are listed from lowest to highest state of understanding:
(2) Prior knowledge,
(1) Descriptive knowledge (what is occurring?),
(3) Design of experiments,
(2) Correlative knowledge (what correlations are empiri-
(4) Simulation of unit operations,
cally observed?),
(5) Selection of a suitable technology platform,
(3) Causal knowledge (empirical, what causes what?),
(6) Mathematical models,
(4) Mechanistic knowledge (explanations for observed
(7) Validated empirical/statistical models,
causality), and
(8) Appropriate instrumentation, and
(5) First principles knowledge (underlying physical,
(9) Appropriate analytical methods.
chemical, and biological phenomena of the mechanistic expla-
4.5.2 The measurement technologies include but are not
nations).
limited to spectroscopic, acoustic, or other rapid sensor tech-
5.2 Process knowledge is the accumulated facts about the
nologies. The development of these and other advanced tech-
process. This accumulated knowledge is generally embodied in
niques will continue to enable or enhance predictive control for
a model of the process. Accordingly, process model is often
commercial pharmaceutical processes.
used synonymously with process knowledge.
4.5.3 The ability to measure process parameters and quality
5.3 Process understanding is demonstrated by the extent to
attributes inline, online, or atline in real time can contribute to
which process knowledge can be used to predict and control
process understanding and the ability to control the process.
the process outcomes; a well understood process will combine
These technologies offer the development scientist, commer-
knowledge from various sources to ensure a well controlled
cial production engineer and manufacturing personnel the
process and consistent product quality.
opportunity for additional insight. This is achieved through the
increased measurement frequency and availability of more 5.4 At any point in time for any manufacturing process, the
comprehensive data. level of understanding will likely be a combination of various
E2475 − 10 (2016)
levels of understanding. As more knowledge is obtained using a risk-based approach, the appropriate level and fre-
throughout the lifecycle of a product, the relative contribution quency of model validation.
to understanding of the various levels is likely to change.
5.12 Periodic evaluation and re-validation (Practice E2617)
5.5 Prior knowledge is any knowledge that may be available
of models should be conducted throughout a product’s life-
through previous experience. Prior knowledge may come from
cycle. This is true from research and development phases and
a number of sources including scientific literature, company
throughout com
...
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E2475 − 10 (Reapproved 2016)
Standard Guide for
Process Understanding Related to Pharmaceutical
Manufacture and Control
This standard is issued under the fixed designation E2475; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope E2474 Practice for Pharmaceutical Process Design Utilizing
Process Analytical Technology (Withdrawn 2020)
1.1 The purpose of this guide is to establish a framework
E2617 Practice for Validation of Empirically Derived Mul-
and context for process understanding for pharmaceutical
2 tivariate Calibrations
manufacturing using quality by design (QbD) (Juran, 1992;
2.2 U.S. Government Publications:
FDA/ICH Q8). The framework is applicable to both active
FDA/ICH Q8 Pharmaceutical Development
pharmaceutical ingredient (API) and to drug product (DP)
FDA/ICH Q10 Pharmaceutical Quality Systems
manufacturing. High (detailed) level process understanding
U.S. FDA PAT Guidance Document, Guidance for Industry
can be used to facilitate production of product which consis-
PAT—A Framework for Innovative Pharmaceutical
tently meets required specifications. It can also play a key role
Manufacturing and Quality Assurance
in continuous process improvement efforts.
1.2 Process Analytical Technology (PAT) is one element 3. Terminology
that can be used for achieving control over those inputs
3.1 Definitions of Terms Specific to This Standard:
determined to be critical to a process. It is important for the
3.1.1 critical inputs, n—critical process parameters and
reader to recognize that PAT is defined as:
critical raw material attributes for a given process.
“{a system for designing, analyzing, and controlling manufacturing through
American Society for Quality
timely measurements (i.e., during processing) of critical quality and performance
3.1.2 empirical, adj—any conclusion based on experimental
attributes of raw and in process materials and processes, with the goal of
ensuring final product quality. It is important to note that the term analytical in
data and past experience, rather than on theory.
PAT is viewed broadly to include chemical, physical, microbiological,
3.1.3 expert system, n—an expert system is a computer
mathematical, and risk analysis conducted in an integrated manner. The goal of
PAT is to enhance understanding and control the manufacturing process{”
program that simulates the judgment and behavior of a human
(U.S. FDA PAT)
or an organization that has expert knowledge and experience in
1.3 This standard does not purport to address all of the
a particular field.
safety concerns, if any, associated with its use. It is the
3.1.3.1 Discussion—Typically, such a system contains a
responsibility of the user of this standard to establish appro-
knowledge base containing accumulated experience and a set
priate safety and health practices and determine the applica-
of rules for applying the knowledge base to each particular
bility of regulatory limitations prior to use.
situation that is described to the program. Sophisticated expert
systems can be enhanced with additions to the knowledge base
2. Referenced Documents
or to the set of rules.
2.1 ASTM Standards:
3.1.4 first principles, n—a calculation is said to be from first
E456 Terminology Relating to Quality and Statistics
principles, or ab initio, if it starts directly at the level of
E2281 Practice for Process Capability and Performance
established laws of physics and does not make assumptions
Measurement
such as model and fitting parameters.
3.1.5 mechanistic, adj—(1) of, or relating to, theories that
This guide is under the jurisdiction of ASTM Committee E55 on Manufacture
explain phenomena in purely physical or deterministic terms: a
of Pharmaceutical and Biopharmaceutical Products and is the direct responsibility of
Subcommittee E55.11 on Process Design.
mechanistic interpretation of nature.
Current edition approved Sept. 1, 2016. Published September 2016. Originally
approved in 2010. Last previous edition approved in 2010 as E2475 – 10.
DOI:10.1520/E2475-10R16. The last approved version of this historical standard is referenced on
Juran, J., Juran on Quality by Design: The New Steps for Planning Quality Into www.astm.org.
Goods and Services, Free Press, New York, N.Y., 1992. Available from U.S. Government Printing Office Superintendent of Documents,
For referenced ASTM standards, visit the ASTM website, www.astm.org, or 732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401, http://
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM www.access.gpo.gov.
Standards volume information, refer to the standard’s Document Summary page on Available from American Society for Quality (ASQ), 600 N. Plankinton Ave.,
the ASTM website. Milwaukee, WI 53203, http://www.asq.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2475 − 10 (2016)
3.1.6 process capability, n—statistical estimate of the out- 4.3.2 Accordingly, the development of process understand-
come of a characteristic from a process that has been demon- ing should be treated as an ongoing process. Learning should
strated to be in a state of statistical control. E2281 continue throughout the product and process life cycle to
improve the level of process understanding to include process
3.1.7 process inputs, n—the combination of all process
parameters and other factors (for example, environmental,
parameters and raw material attributes for a given process.
changes of scale, changes in raw materials, changes in person-
3.1.8 process understanding, v—to recall and comprehend
nel) which may have changed or which may have newly
process knowledge such that product quality can be explained
emerged since the time the process was first commissioned.
logically or scientifically, or both, as a function of process
Work to enhance process understanding continuously through-
inputs and respond accordingly.
out the life cycle of the product and process can provide
3.1.9 residual error, n—the difference between the observed assurance that the process will continue to have an acceptably
low risk of producing out of specification results.
result and the predicted value (estimated treatment response);
Observed Result minus Predicted Value. E456 4.3.3 Manufacturers are encouraged to continuously moni-
tor and improve upon their operations to enhance product
3.1.10 uncertainty, n—an indication of the variability asso-
quality.
ciated with a measured value that takes into account two major
components of error: (1) bias, and (2) the random error 4.4 Process Understanding for the Whole Process:
attributed to the imprecision of the measurement process. E456 4.4.1 For each product, process understanding covers the
process from the initial design of the chemical or biological
4. Process Understanding drug substance through manufacturing of the unit dose or
device to final packaging. In addition, the critical quality
4.1 From physical, chemical, biological, and microbiologi-
attributes of the raw materials will in turn become inputs to the
cal perspectives, a process is considered to be well understood
drug product manufacturing process, as will process param-
when:
eters.
(1) All significant sources of variability in process inputs
4.4.2 Fig. 1 schematically illustrates that the performance of
are identified and explained,
any process output (Y) is a function of the inputs (X), which can
(2) The effect of these sources of variability on product
be classified into one of six categories (that is, operator,
quality attributes can be accurately and reliably estimated
equipment, measurements, methods, materials, and environ-
based on the inputs to the process, and
mental conditions).
(3) Significant process parameters are continuously man-
4.4.3 Comprehensive understanding of the relationships of
aged and controlled to ensure that the process must produce
the process inputs and operating parameters to quality attri-
product which is continuously within required specifications to
butes of the resulting product is fundamental to developing a
the user specified required degree or confidence.
successful risk mitigation or control strategy, or both. Identi-
4.2 A well-controlled process is a process where the risk of
fication of critical process parameters (CPPs) and critical raw
producing product not meeting required specifications is below
material attributes should be carried out using suitable experi-
the maximum acceptable level of risk as predetermined by the
mental and investigative techniques. An understanding of these
user. Accordingly, process understanding requires the compre-
critical inputs (CPPs and critical raw material attributes), and
hension and recall of process knowledge sufficient for the
their monitoring and control, is essential when designing a
logical, statistical, or scientific understanding, or combination
process that is able to consistently and reliably deliver product
thereof, of how significant process parameters and raw material
of the desired quality.
attributes relate to, or impact the quality attributes of, the
4.4.4 One method for achieving the desired state is through
product being produced. Sufficient process understanding
multivariate analysis and control. The acceptable operating
should be achieved to reduce risk to an acceptable level for the
envelope of the critical inputs defines the relationship between
patient, manufacturer, or any other stakeholder.
the design space, control strategy and operating range(s).
4.4.5 Note that for raw materials, an additional source of
4.3 A Lifecycle Commitment (Development and Commercial
variability derives from the potential for adulteration. This
Manufacture):
requires that manufacturers understand their incoming supply
4.3.1 Process understanding is fundamental to QbD. It is
chain and suppliers quality systems, and include methods to
important to realize that due to commercial realities (for
detect adulteration of materials in addition to confirming
example, finite resources, time, and money), a process will
identity as necessary, bearing in mind that adulteration may be
typically be commissioned as soon as the degree of process
difficult to detect by standard methods. It also requires that
understanding is sufficient to permit operation of the process
manufacturers use suppliers that are aware of these concerns
with an acceptably low, user specified, level of risk of
and are prepared to implement their own precautionary
producing out of specification product. While it may be
measures, and to permit transparency into their respective
appropriate to commission a process once this minimum
supply sources.
degree of process understanding is achieved, the risk that the
process may transition out of control steadily increases over 4.5 Tools of Process Understanding:
time (for example, process drift), and could exceed the 4.5.1 Process understanding begins with process design
maximum acceptable risk without warning, unless an ongoing (Practice E2474) and usually a structured, small scale devel-
program to enhance process understanding is in place. opment program which focuses on efficiently delivering a
E2475 − 10 (2016)
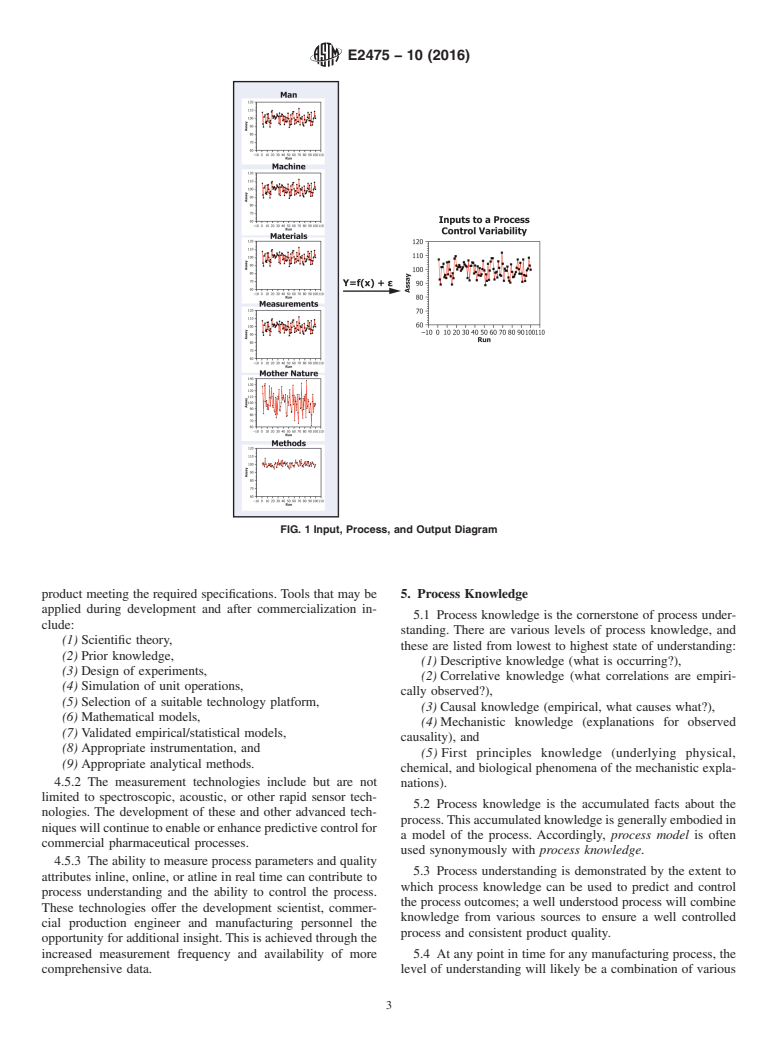
FIG. 1 Input, Process, and Output Diagram
product meeting the required specifications. Tools that may be 5. Process Knowledge
applied during development and after commercialization in-
5.1 Process knowledge is the cornerstone of process under-
clude:
standing. There are various levels of process knowledge, and
(1) Scientific theory,
these are listed from lowest to highest state of understanding:
(2) Prior knowledge,
(1) Descriptive knowledge (what is occurring?),
(3) Design of experiments,
(2) Correlative knowledge (what correlations are empiri-
(4) Simulation of unit operations,
cally observed?),
(5) Selection of a suitable technology platform,
(3) Causal knowledge (empirical, what causes what?),
(6) Mathematical models,
(4) Mechanistic knowledge (explanations for observed
(7) Validated empirical/statistical models,
causality), and
(8) Appropriate instrumentation, and
(5) First principles knowledge (underlying physical,
(9) Appropriate analytical methods.
chemical, and biological phenomena of the mechanistic expla-
4.5.2 The measurement technologies include but are not
nations).
limited to spectroscopic, acoustic, or other rapid sensor tech-
5.2 Process knowledge is the accumulated facts about the
nologies. The development of these and other advanced tech-
process. This accumulated knowledge is generally embodied in
niques will continue to enable or enhance predictive control for
a model of the process. Accordingly, process model is often
commercial pharmaceutical processes.
used synonymously with process knowledge.
4.5.3 The ability to measure process parameters and quality
5.3 Process understanding is demonstrated by the extent to
attributes inline, online, or atline in real time can contribute to
which process knowledge can be used to predict and control
process understanding and the ability to control the process.
the process outcomes; a well understood process will combine
These technologies offer the development scientist, commer-
knowledge from various sources to ensure a well controlled
cial production engineer and manufacturing personnel the
process and consistent product quality.
opportunity for additional insight. This is achieved through the
increased measurement frequency and availability of more 5.4 At any point in time for any manufacturing process, the
comprehensive data. level of understanding will likely be a combination of various
E2475 − 10 (2016)
levels of understanding. As more knowledge is obtained using a risk-based approach, the appropriate level and fre-
throughout the lifecycle of a product, the relative contribution quency of model validation.
to understanding of the various levels is likely to change.
5.12 Periodic evaluation and re-validation (Practice E2617)
5.5 Prior knowledge is any knowledge that may be available
of models should be conducted throughout a product’s life-
through previous experience. Prior knowledge may come from
cycle. This is true from research and development phases and
a number of sources including scientific literature, company
throughout commercialization of a product, where additional
experience from research and development, and existing com-
data (for example,
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E2475 − 10 E2475 − 10 (Reapproved 2016)
Standard Guide for
Process Understanding Related to Pharmaceutical
Manufacture and Control
This standard is issued under the fixed designation E2475; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 The purpose of this guide is to establish a framework and context for process understanding for pharmaceutical
manufacturing using quality by design (QbD) (Juran, 1992; FDA/ICH Q8). The framework is applicable to both active
pharmaceutical ingredient (API) and to drug product (DP) manufacturing. High (detailed) level process understanding can be used
to facilitate production of product which consistently meets required specifications. It can also play a key role in continuous process
improvement efforts.
1.2 Process Analytical Technology (PAT) is one element that can be used for achieving control over those inputs determined
to be critical to a process. It is important for the reader to recognize that PAT is defined as:
“{a system for designing, analyzing, and controlling manufacturing through timely measurements (i.e., during processing)
of critical quality and performance attributes of raw and in process materials and processes, with the goal of ensuring final
product quality. It is important to note that the term analytical in PAT is viewed broadly to include chemical, physical,
microbiological, mathematical, and risk analysis conducted in an integrated manner. The goal of PAT is to enhance
understanding and control the manufacturing process{” (U.S. FDA PAT)
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
E456 Terminology Relating to Quality and Statistics
E2281 Practice for Process Capability and Performance Measurement
E2474 Practice for Pharmaceutical Process Design Utilizing Process Analytical Technology
E2617 Practice for Validation of Empirically Derived Multivariate Calibrations
2.2 U.S. Government Publications:
FDA/ICH Q8 Pharmaceutical Development
FDA/ICH Q10 Pharmaceutical Quality Systems
U.S. FDA PAT Guidance Document, Guidance for Industry PAT—A Framework for Innovative Pharmaceutical Manufacturing
and Quality Assurance
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 critical inputs, n—critical process parameters and critical raw material attributes for a given process.
American Society for Quality
3.1.2 empirical, adj—any conclusion based on experimental data and past experience, rather than on theory.
This guide is under the jurisdiction of ASTM Committee E55 on Manufacture of Pharmaceutical and Biopharmaceutical Products and is the direct responsibility of
Subcommittee E55.01 on Process Understanding and PAT System Management, Implementation and Practice.
Current edition approved April 15, 2010Sept. 1, 2016. Published August 2010September 2016. DOI:10.1520/E2475-10.Originally approved in 2010. Last previous edition
approved in 2010 as E2475 – 10. DOI:10.1520/E2475-10R16.
Juran, J., Juran on Quality by Design: The New Steps for Planning Quality Into Goods and Services, Free Press, New York, N.Y., 1992.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Available from U.S. Government Printing Office Superintendent of Documents, 732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401, http://
www.access.gpo.gov.
Available from American Society for Quality (ASQ), 600 N. Plankinton Ave., Milwaukee, WI 53203, http://www.asq.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2475 − 10 (2016)
3.1.3 expert system, n—an expert system is a computer program that simulates the judgment and behavior of a human or an
organization that has expert knowledge and experience in a particular field.
3.1.3.1 Discussion—
Typically, such a system contains a knowledge base containing accumulated experience and a set of rules for applying the
knowledge base to each particular situation that is described to the program. Sophisticated expert systems can be enhanced with
additions to the knowledge base or to the set of rules.
3.1.4 first principles, n—a calculation is said to be from first principles, or ab initio, if it starts directly at the level of established
laws of physics and does not make assumptions such as model and fitting parameters.
3.1.5 mechanistic, adj—(1) of, or relating to, theories that explain phenomena in purely physical or deterministic terms: a
mechanistic interpretation of nature.
3.1.6 process capability, n—statistical estimate of the outcome of a characteristic from a process that has been demonstrated to
be in a state of statistical control. E2281
3.1.7 process inputs, n—the combination of all process parameters and raw material attributes for a given process.
3.1.8 process understanding, v—to recall and comprehend process knowledge such that product quality can be explained
logically or scientifically, or both, as a function of process inputs and respond accordingly.
3.1.9 residual error, n—the difference between the observed result and the predicted value (estimated treatment response);
Observed Result minus Predicted Value. E456
3.1.10 uncertainty, n—an indication of the variability associated with a measured value that takes into account two major
components of error: (1) bias, and (2) the random error attributed to the imprecision of the measurement process. E456
4. Process Understanding
4.1 From physical, chemical, biological, and microbiological perspectives, a process is considered to be well understood when:
(1) All significant sources of variability in process inputs are identified and explained,
(2) The effect of these sources of variability on product quality attributes can be accurately and reliably estimated based on the
inputs to the process, and
(3) Significant process parameters are continuously managed and controlled to ensure that the process must produce product
which is continuously within required specifications to the user specified required degree or confidence.
4.2 A well-controlled process is a process where the risk of producing product not meeting required specifications is below the
maximum acceptable level of risk as predetermined by the user. Accordingly, process understanding requires the comprehension
and recall of process knowledge sufficient for the logical, statistical, or scientific understanding, or combination thereof, of how
significant process parameters and raw material attributes relate to, or impact the quality attributes of, the product being produced.
Sufficient process understanding should be achieved to reduce risk to an acceptable level for the patient, manufacturer, or any other
stakeholder.
4.3 A Lifecycle Commitment (Development and Commercial Manufacture):
4.3.1 Process understanding is fundamental to QbD. It is important to realize that due to commercial realities (for example, finite
resources, time, and money), a process will typically be commissioned as soon as the degree of process understanding is sufficient
to permit operation of the process with an acceptably low, user specified, level of risk of producing out of specification product.
While it may be appropriate to commission a process once this minimum degree of process understanding is achieved, the risk that
the process may transition out of control steadily increases over time (for example, process drift), and could exceed the maximum
acceptable risk without warning, unless an ongoing program to enhance process understanding is in place.
4.3.2 Accordingly, the development of process understanding should be treated as an ongoing process. Learning should continue
throughout the product and process life cycle to improve the level of process understanding to include process parameters and other
factors (for example, environmental, changes of scale, changes in raw materials, changes in personnel) which may have changed
or which may have newly emerged since the time the process was first commissioned. Work to enhance process understanding
continuously throughout the life cycle of the product and process can provide assurance that the process will continue to have an
acceptably low risk of producing out of specification results.
4.3.3 Manufacturers are encouraged to continuously monitor and improve upon their operations to enhance product quality.
4.4 Process Understanding for the Whole Process:
4.4.1 For each product, process understanding covers the process from the initial design of the chemical or biological drug
substance through manufacturing of the unit dose or device to final packaging. In addition, the critical quality attributes of the raw
materials will in turn become inputs to the drug product manufacturing process, as will process parameters.
E2475 − 10 (2016)
4.4.2 Fig. 1 schematically illustrates that the performance of any process output (Y) is a function of the inputs (X), which can
be classified into one of six categories (that is, operator, equipment, measurements, methods, materials, and environmental
conditions).
4.4.3 Comprehensive understanding of the relationships of the process inputs and operating parameters to quality attributes of
the resulting product is fundamental to developing a successful risk mitigation or control strategy, or both. Identification of critical
process parameters (CPPs) and critical raw material attributes should be carried out using suitable experimental and investigative
techniques. An understanding of these critical inputs (CPPs and critical raw material attributes), and their monitoring and control,
is essential when designing a process that is able to consistently and reliably deliver product of the desired quality.
4.4.4 One method for achieving the desired state is through multivariate analysis and control. The acceptable operating
rangeenvelope of the critical inputs defines the relationship between the design space, control strategy and operating range(s).
4.4.5 Note that for raw materials, an additional source of variability derives from the potential for adulteration. This requires
that manufacturers understand their incoming supply chain and suppliers quality systems, and include methods to detect
adulteration of materials in addition to confirming identity as necessary, bearing in mind that adulteration may be difficult to detect
by standard methods. It also requires that manufacturers use suppliers that are aware of these concerns and are prepared to
implement their own precautionary measures, and to permit transparency into their respective supply sources.
4.5 Tools of Process Understanding:
4.5.1 Process understanding begins with process design (Practice E2474) and usually a structured, small scale development
program which focuses on efficiently delivering a product meeting the required specifications. Tools that may be applied during
development and after commercialization include:
(1) Scientific theory,
(2) Prior knowledge,
(3) Design of experiments,
(4) Simulation of unit operations,
(5) Selection of a suitable technology platform,
(6) Mathematical models,
(7) Empirical/statistical Validated empirical/statistical models,
(8) Appropriate instrumentation, and
FIG. 1 Input, Process, and Output Diagram
E2475 − 10 (2016)
(9) Appropriate analytical methods.
4.5.2 The measurement technologies include but are not limited to spectroscopic, acoustic, or other rapid sensor technologies.
The development of these and other advanced techniques will continue to enable or enhance predictive control for commercial
pharmaceutical processes.
4.5.3 The ability to measure process parameters and quality attributes inline, online, or atline in real time can contribute to
process understanding and the ability to control the process. These technologies offer the development scientist, commercial
production engineer and manufacturing personnel the opportunity for additional insight. This is achieved through the increased
measurement frequency and availability of more comprehensive data.
5. Process Knowledge
5.1 Process knowledge is the cornerstone of process understanding. There are various levels of process knowledge, and these
are listed from lowest to highest state of understanding:
(1) Descriptive knowledge (what is occurring?),
(2) Correlative knowledge (what correlations are empirically observed?),
(3) Causal knowledge (empirical, what causes what?),
(4) Mechanistic knowledge (explanations for observed causality), and
(5) First principles knowledge (underlying physical, chemical, and biological phenomena of the mechanistic explanations).
5.2 Process knowledge is the accumulated facts about the process. This accumulated knowledge is generally embodied in a
model of the process. Accordingly, process model is often used synonymously with process knowledge.
5.3 Process understanding is demonstrated by the extent to which process knowledge can be used to predict and control the
process outcomes; a well understood process will combine knowledge from various sources to ensure a well controlled process
and consistent product quality.
5.4 At any point in time for any manufacturing process, the level of understanding will likely be a combination of various levels
of understanding. As more knowledge is obtained throughout the lifecycle of a product, the relative contribution to understanding
of the various levels is likely to change.
5.5 Prior knowledge is any knowledge that may be available through previous experience. Prior knowledge may come from a
number of sources including scientific literature, company experience from research and development, and existing commercial
products such as as a result of lab and manufacturing investigations. All knowledge that is available shoul
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.