ASTM F1524-95
(Guide)Standard Guide for Use of Advanced Oxidation Process for the Mitigation of Chemical Spills
Standard Guide for Use of Advanced Oxidation Process for the Mitigation of Chemical Spills
SCOPE
1.1 This guide covers the considerations for advanced oxidation processes (AOPs) in the mitigation of spilled chemicals and hydrocarbons dissolved into ground and surface waters.
1.2 This guide addresses the application of advanced oxidation alone or in conjunction with other technologies.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. In addition, it is the responsibility of the user to ensure that such activity takes place under the control and direction of a qualified person with full knowledge of any potential safety and health protocols.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 1524 – 95
Standard Guide for
Use of Advanced Oxidation Process for the Mitigation of
Chemical Spills
This standard is issued under the fixed designation F 1524; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope with hydroxyl radicals that do not yield species that propagate
the chain reaction for contaminant destruction. Scavengers can
1.1 This guide covers the considerations for advanced
be either organic or inorganic compounds.
oxidation processes (AOPs) in the mitigation of spilled chemi-
cals and hydrocarbons dissolved into ground and surface
3. Significance and Use
waters.
3.1 General—This guide contains information regarding the
1.2 This guide addresses the application of advanced oxi-
use of AOPs to oxidize and eventually mineralize hazardous
dation alone or in conjunction with other technologies.
materials that have entered surface and groundwater as the
1.3 This standard does not purport to address all of the
result of a spill. Since much of this technology development is
safety concerns, if any, associated with its use. It is the
still at the benchscale level, these guidelines will only refer to
responsibility of the user of this standard to establish appro-
those units that are currently applied at a field scale level.
priate safety and health practices and determine the applica-
3.2 Oxidizing Agents:
bility of regulatory limitations prior to use. In addition, it is the
3.2.1 Hydroxyl Radical (OH)—The OH radical is the most
responsibility of the user to ensure that such activity takes
common oxidizing agent employed by this technology due to
place under the control and direction of a qualified person with
its powerful oxidizing ability. When compared to other oxi-
full knowledge of any potential safety and health protocols.
dants such as molecular ozone, hydrogen peroxide, or hy-
2. Terminology pochlorite, its rate of attack is commonly much faster. In fact,
6 9
it is typically one million (10 ) to one billion (10 ) times faster
2.1 Definitions of Terms Specific to This Standard:
than the corresponding attack with molecular ozone (1). The
2.1.1 advanced oxidation processes (AOPs)—ambient tem-
three most common methods for generating the hydroxyl
perature processes that involve the generation of highly reac-
radical are described in the following equations:
tive radical species and lead to the oxidation of waterborne
contaminants (usually organic) in surface and ground waters. H O 1 hv → 2OH· (1)
2 2
2.1.2 inorganic foulants—compounds, such as iron, calcium
2O 1 H O →→ 2OH· 1 3O (2)
3 2 2 2
and manganese, that precipitate throughout a treatment unit
12 13 2
Fe 1 H O →→ OH·Fe 1 OH Fenton’s Reaction (3)
~ !
2 2
and cause reduced efficiency by fouling the quartz sleeve that
3.2.1.1 Hydrogen peroxide is the preferred oxidant for
protects the lamp in photolytic oxidation AOP systems or the
photolytic oxidation systems since ozone will encourage the air
fibreglass mesh that is coated with TiO in photocatalytic AOP
stripping of solutions containing volatile organics (2). Capital
systems.
and operating costs are also taken into account when a decision
2.1.3 mineralization—the complete oxidation of an organic
on the choice of oxidant is made.
compound to carbon dioxide, water, and acid compounds, that
3.2.1.2 Advanced oxidation technology has also been devel-
is, hydrochloric acid if the compound is chlorinated.
oped based on the anatase form of titanium dioxide. This
2.1.4 photoreactor—the core of the photoreactor is a UV
method by which the photocatalytic process generates hy-
lamp that emits light in the broad range of 200 to 400 nm
droxyl radicals is described in the following equations:
wavelength range.
2.1.5 radical species—a powerful oxidizing agent, princi- 1 2
TiO 1 hv 1 H O → OH· 1 H 1 e (4)
2 2
pally the hydroxyl radical, that reacts rapidly with virtually all
2 2
2e 1 2O 1 2H O→ 2OH· 1 O 1 2OH (5)
2 2 2
organic compounds to oxidize and eventually lead to their
complete mineralization.
3.2.2 Photolysis—Destruction pathways, besides the hy-
2.1.6 scavengers—a term used for substances that react droxyl radical attack, are very important for the more refrac-
tory compounds such as chloroform, carbon tetrachloride,
This guide is under the jurisdiction of ASTM Committee F-20 on Hazardous
Substances and Oil Spill Response and is the direct responsibility of Subcommittee
F20.22 on Mitigation Actions.
Current edition approved May 15, 1995. Published July 1995. Originally The boldface numbers in parentheses refer to the list of references at the end of
published as F 1524 – 94. Last previous edition F 1524 – 94. this standard.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
F 1524
trichloroethane, and other chlorinated methane or ethane com- trations where the inorganics could be separated and removed.
pounds. A photoreactor’s ability to destroy these compounds 4.5 System Fouling—Generally, inorganic foulants, such as
photochemically will depend on its output level at specific iron, manganese, and calcium, in the ppm range, cause reduced
wavelengths. Since most of these lamps are proprietary, flow, increased pressure and low performance of a treatment
preliminary benchscale testing becomes crucial when dealing system. This phenomenon is common in most organic treat-
with these compounds. ment units regardless of the mechanism employed. Pretreat-
3.3 AOP Treatment Techniques: ment systems usually involve chemical addition (that is, pH
3.3.1 Advanced oxidation processes (AOPs) may be applied adjustment) or membrane technology, or both, as they are
alone or in conjunction with other treatment techniques as generally the most economical and effective for inorganic
follows: removal. Preliminary benchscale testing is commonly used to
3.3.1.1 Following a pretreatment step. The pretreatment determine the applicability and the cost-effectiveness of the
process can be either a physical or chemical process for the different pretreatment systems.
removal of inorganic or organic scavengers from the contami- 4.6 Off-Gas Analysis—Organic analysis of the exiting gas-
nated stream prior to AOP destruction. eous stream will assist the operator in modifying system
3.3.1.2 Following a preconcentration step. Due to the in- parameters to maximize system performance and efficiency.
crease in likelihood of radical or molecule contact, very dilute This technique is also beneficial during preliminary testing as
solutions can be treated cost effectively using AOPs after being it provides an indication of the AOP technology’s ability to
concentrated. destroy the compounds as compared to simply stripping them
3.4 AOP Treatment Applications—Advanced oxidation pro- from the water phase into the air.
cesses (AOPs) are most cost effective for those waste streams 4.7 Destruction Rate Constants—The reaction of the OH
containing organic compounds at concentrations below 1 % radical with organic compounds is largely dependent upon the
(10 000 ppm). This figure will vary depending upon the nature rate constant. A list (3) of reaction rates for common contami-
of the compounds and whether there is competition for the nants is shown in Table 1.
oxidizing agent.
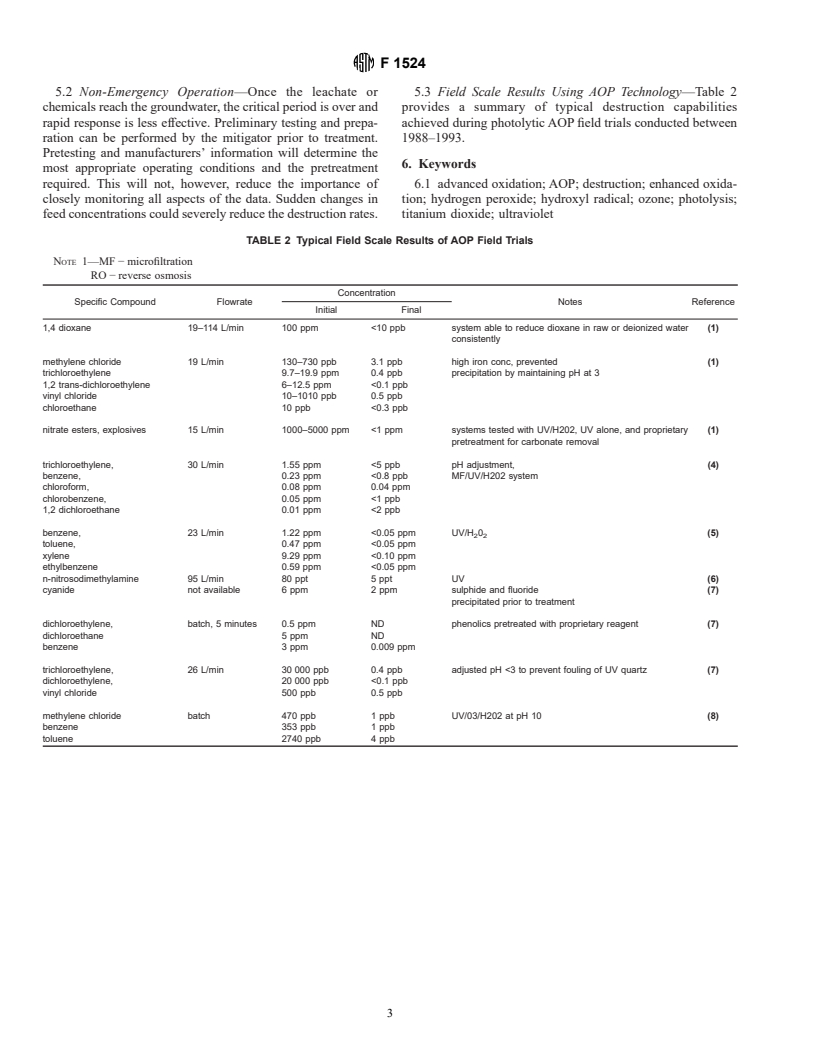
5. Practical Applications
4. Constraints on Usage
5.1 Emergency Situations—Advanced oxidation process
(AOP) applications would normally follow containment and
4.1 General—Although AOPs are destruction processes, in
recovery of the waste stream in question. The time required for
order for compound mineralization to take place, the oxidation
this primary stage should be sufficient for the AOP user to at
reactions must be taken to completion. In most cases, effluent
least obtain the n
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.