ASTM D2274-03a(2008)
(Test Method)Standard Test Method for Oxidation Stability of Distillate Fuel Oil (Accelerated Method)
Standard Test Method for Oxidation Stability of Distillate Fuel Oil (Accelerated Method)
SIGNIFICANCE AND USE
This test method provides a basis for the estimation of the storage stability of middle distillate fuels such as No. 2 fuel oil.
The test method may not provide a prediction of the quantity of insolubles that will form in field storage over any given period of time. The amount of insolubles formed in such field storage is subject to the specific conditions which are too variable for this test method to predict accurately.
Test Method D 2274 yields results more rapidly than Test Method D 4625, the 43°C bottle test. However, as a result of the significantly elevated temperature and the pure oxygen atmosphere, the nature and amount of insolubles may deviate to a greater extent than Test Method D 4625 from those formed in field storage.
SCOPE
1.1 This test method covers the measurement of the inherent stability of middle distillate petroleum fuels under specified oxidizing conditions at 95°C.
Note 1—Fuels used in establishing the precision measures for this test method were described as gas oil, diesel fuel, No. 2 heating oil, and DFM, a Navy distillate fuel suitable for diesels, boilers, and gas turbines. (The term DFM is no longer used when referring to fuel meeting MIL-F-16884 requirements; rather it is called F76 as it conforms to NATO F76 requirements.) While the test method may be used for fuels outside the range of these fuels, the precision measures may not apply.
1.2 This test method is not applicable to fuels containing residual oil or significant amounts of components derived from non-petroleum sources.
1.3 The values given in acceptable SI units are to be regarded as the standard. The values in parentheses are for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D2274–03a (Reapproved 2008)
Designation:388/97
Standard Test Method for
Oxidation Stability of Distillate Fuel Oil (Accelerated
Method)
This standard is issued under the fixed designation D2274; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope D4057 Practice for Manual Sampling of Petroleum and
Petroleum Products
1.1 Thistestmethodcoversthemeasurementoftheinherent
D4177 Practice for Automatic Sampling of Petroleum and
stability of middle distillate petroleum fuels under specified
Petroleum Products
oxidizing conditions at 95°C.
D4625 Test Method for Middle Distillate Fuel Storage
NOTE 1—Fuels used in establishing the precision measures for this test
Stability at 43°C (110°F)
method were described as gas oil, diesel fuel, No. 2 heating oil, and DFM,
2.2 Military Specification:
a Navy distillate fuel suitable for diesels, boilers, and gas turbines. (The
MIL-F-16884 Fuel, Navy Distillate
term DFM is no longer used when referring to fuel meeting MIL-F-16884
requirements; rather it is called F76 as it conforms to NATO F76
3. Terminology
requirements.) While the test method may be used for fuels outside the
range of these fuels, the precision measures may not apply. 3.1 Definitions of Terms Specific to This Standard:
3.1.1 adherent insolubles (formerly adherent gum)—
1.2 This test method is not applicable to fuels containing
material which is produced in the course of stressing distillate
residual oil or significant amounts of components derived from
fuel under the conditions of this test and which adheres to the
non-petroleum sources.
glassware after fuel has been flushed from the system.
1.3 The values given in acceptable SI units are to be
3.1.2 filterable insolubles—material, which is produced in
regarded as the standard. The values in parentheses are for
the course of stressing distillate fuel under the conditions of
information only.
this test, which is capable of being removed from the fuel by
1.4 This standard does not purport to address all of the
filtration.This includes both material suspended in the fuel and
safety concerns, if any, associated with its use. It is the
material easily removed from the oxidation cell and oxygen
responsibility of the user of this standard to establish appro-
delivery tube with hydrocarbon solvent.
priate safety and health practices and determine the applica-
3.1.3 inherent stability—the resistance to change when
bility of regulatory limitations prior to use.
exposed to air, but in the absence of other environmental
2. Referenced Documents factors such as water, or reactive metallic surfaces and dirt.
3.1.4 total insolubles—sum of the adherent and filterable
2.1 ASTM Standards:
insolubles.
D381 Test Method for Gum Content in Fuels by Jet Evapo-
3.1.5 zero time—the time the first of a batch of oxidation
ration
cells is placed in the heating bath.
D943 Test Method for Oxidation Characteristics of Inhib-
3.1.5.1 Discussion—This is the time taken as the start of the
ited Mineral Oils
16 h of residence in the heating bath.
D1193 Specification for Reagent Water
4. Summary of Test Method
This test method is under the jurisdiction of Committee D02 on Petroleum
4.1 A 350-mL volume of filtered middle distillate fuel is
Products and Lubricants and is the direct responsibility of Subcommittee D02.14 on
agedat95°C(203°F)for16hwhileoxygenisbubbledthrough
Stability and Cleanliness of Liquid Fuels.
the sample at a rate of 3 L/h.After aging, the sample is cooled
Current edition approved Dec. 1, 2008. Published February 2009. Originally
approved in 1964. Last previous edition approved in 2003 as D2274–03a. DOI: to approximately room temperature before filtering to obtain
10.1520/D2274-03AR08.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on Available from Standardization Documents Order Desk, Bldg. 4, 700 Robbins
the ASTM website. Ave., Philadelphia, PA 19111-5098. Attn: NPODS
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D2274–03a (2008)
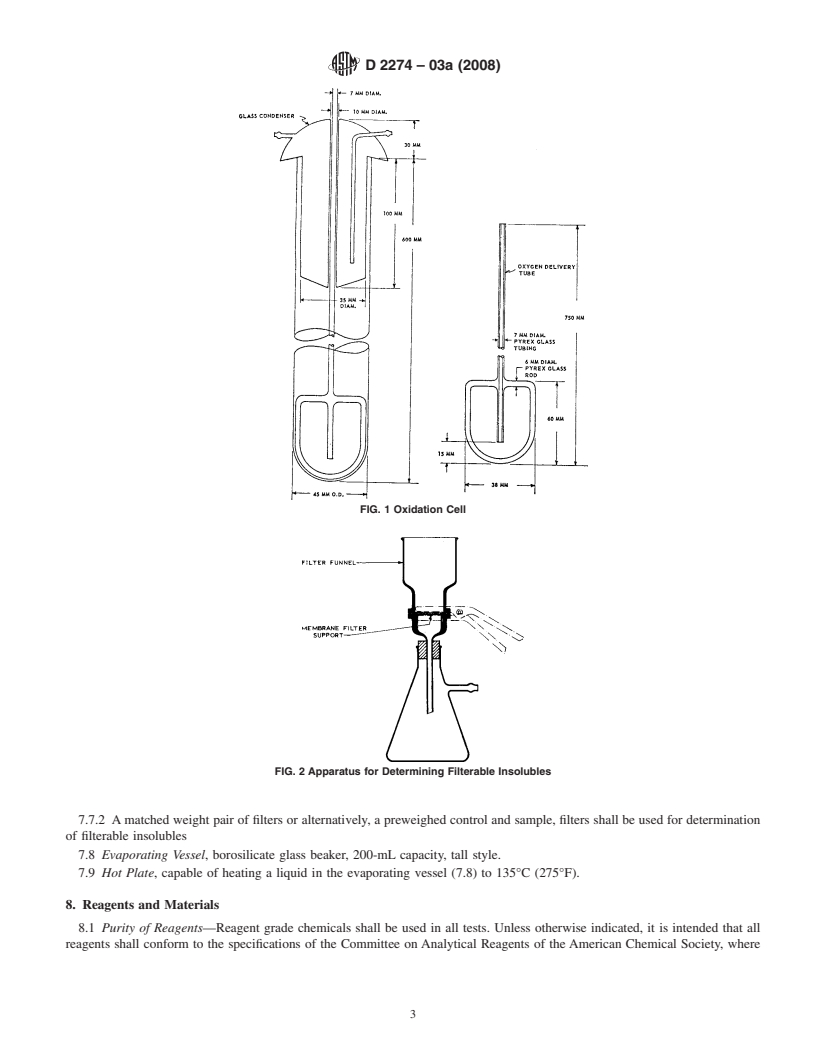
FIG. 1 Oxidation Cell
the filterable insolubles quantity. Adherent insolubles are then coils of copper and steel are used, it is important that any
removed from the oxidation cell and associated glassware with residues that could contain these metals be eliminated from the
trisolvent.The trisolvent is evaporated to obtain the quantity of apparatus by thorough cleaning prior to use. Similarly, to
adherent insolubles. The sum of the filterable and adherent preclude the presence of chromium ions, as well as to protect
insolubles, expressed as milligrams per 100 mL, is reported as laboratory personnel from potential harm, chromic acid shall
total insolubles. not be used for cleaning glassware in the practice of this
method.
5. Significance and Use
6.2 It has been found that commercial grades of acetone, if
5.1 This test method provides a basis for the estimation of
used in the trisolvent, can have impurities which cause an
the storage stability of middle distillate fuels such as No. 2 fuel
apparently greater level of adherent insolubles to be measured.
oil.
It is, therefore, critical that only reagent (or higher) grade
5.2 The test method may not provide a prediction of the
materials be used in preparing the trisolvent mixture.
quantity of insolubles that will form in field storage over any
6.3 Ultravioletlightexposurehasbeenfoundtoincreasethe
given period of time. The amount of insolubles formed in such
amount of total insolubles. Therefore, the fuel being tested
field storage is subject to the specific conditions which are too
shall be shielded from direct exposure to ultraviolet light
variable for this test method to predict accurately.
(sunlight or fluorescent). Conduct all sampling, measuring,
5.3 TestMethodD2274yieldsresultsmorerapidlythanTest
filtration, and weighing away from direct sunlight and in as
MethodD4625,the43°Cbottletest.However,asaresultofthe
dark an area as would be compatible with other laboratory
significantly elevated temperature and the pure oxygen atmo-
operations. Storage before stress, the stress period and cool-
sphere, the nature and amount of insolubles may deviate to a
down after stressing shall be in the dark.
greater extent than Test Method D4625 from those formed in
field storage.
7. Apparatus
6. Interferences
NOTE 2—It is suggested that all equipment be calibrated according to
manufacturer’s instructions on a periodic basis to assure consistency of
6.1 Oxidation is a major chemical process causing adherent
results.
and filterable insolubles to form.Any substance such as copper
or chromium that catalyzes oxidation reactions will cause 7.1 Oxidation Cell, of borosilicate glass, as shown in Fig. 1,
greater quantities of insolubles to form. Since the apparatus shall consist of a test tube, condenser, and oxygen delivery
used in this test can also be used in Test Method D943, where tube. This cell is identical to that used in Test Method D943.
D2274–03a (2008)
where such specifications are available. Other grades may be
used, provided it is first ascertained that the reagent is of
sufficiently high purity to permit its use without lessening the
accuracy of the determination.
8.2 Purity of Water—Unless otherwise indicated, reference
to water shall be understood to mean reagent water as defined
by Type III of Specification D1193.
8.3 2.2,4-trimethylpentanel (isooctane), 99.75 % purity pre-
filtered through a filter medium of the type specified in 7.7.
8.4 Oxygen, 99.5 % purity or better. When the oxygen is
delivered through a plant system of piping, a filter shall be
provided adjacent to the constant temperature bath to prevent
the introduction of line debris or moisture into the oxidation
cells; a pressure regulator adequate to maintain a constant flow
of gas through the apparatus shall also be used. A tank of
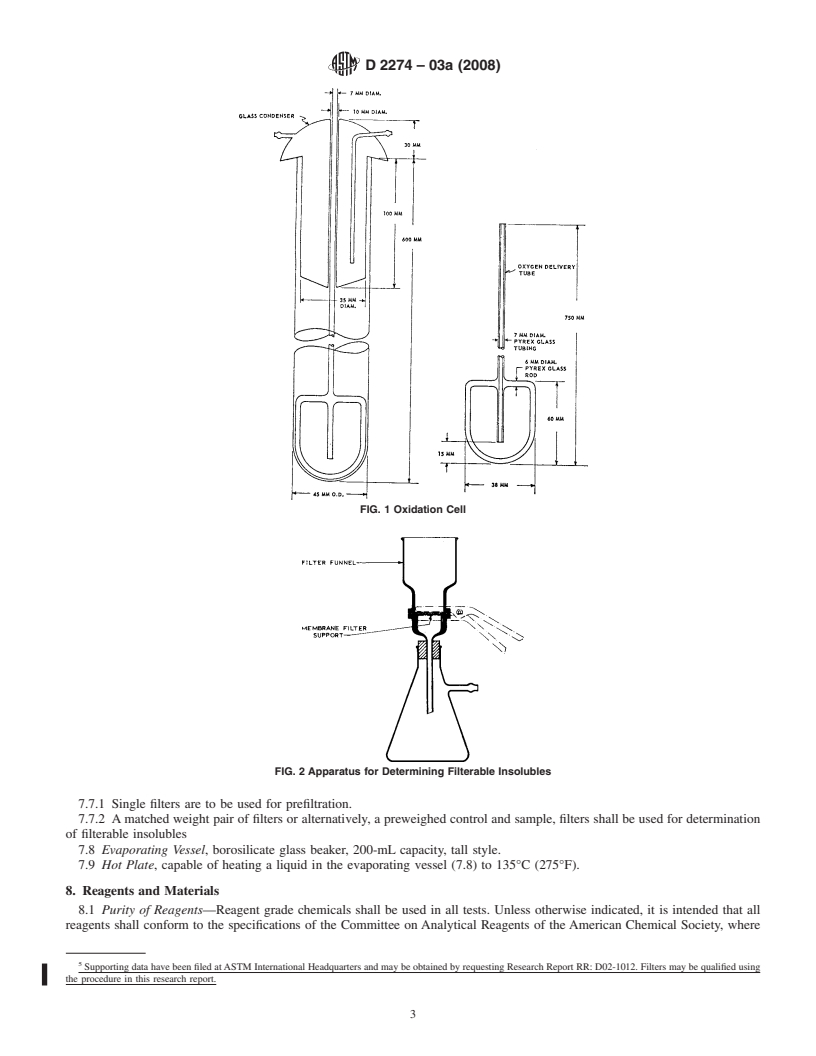
FIG. 2 Apparatus for Determining Filterable Insolubles oxygen of the specified purity can be used provided it is
equipped with a two-stage pressure regulator. (Warning—
Oxygen vigorously accelerates combustion. Do not use equip-
7.2 Heating Bath, with a thermostatically controlled liquid
ment having exposed surfaces containing oil or grease.)
medium, shall be capable of maintaining the bath temperature
8.5 Trisolvent, a mixture of equal volumes of acetone,
at 95 6 0.2°C (203 6 0.4°F). It shall be fitted with a suitable
methanol, and toluene. See 8.1.(Warning—It is particularly
stirringdevicetoprovideauniformtemperaturethroughoutthe
important that technical, commercial, practical, or industrial
bath. It shall be large enough to hold the desired number of
grades (however they are designated by the particular manu-
oxidation cells immersed to a depth of approximately 350 mm.
facturer) are not to be used, as their use may lead to apparently
Further, the bath construction must permit shielding the fuel
increased levels of adherent insolubles.) (Warning—Fire haz-
samples in the oxidation cells from light while they are
ard, toxic.)
undergoing oxidation.
7.3 Flowmeters, shall have a capability of measuring 3 6
9. Samples and Sampling
0.3 L/h of oxygen. One flowmeter shall be provided for each
9.1 When obtaining samples for the laboratory, follow
oxidation cell.
Practices D4057 or D4177, or other standard practice capable
7.4 Filter Drying Oven, shall be capable of safely evapo-
of providing representative samples.
rating the solvent at 80° 6 2°C (176° 6 4°F) for the drying of
9.2 Analyze fuel samples as soon as possible after receipt.
filter materials.
When a fuel cannot be tested within one day, blanket it with an
7.5 Glassware Drying Oven, shall be capable of drying
inert gas such as oxygen-free nitrogen, argon, or helium and
glassware at 105° 6 5°C (221 6 9°F).
storeatatemperaturenohigherthan10°C(50°F)butnotlower
7.6 Filter Assembly, see Fig. 2, shall be capable of holding
than the cloud point. (Warning—Plastic containers are not
the filters described in 7.7.
acceptable for samples due to the potential for leaching of
7.7 Filter Media , 47 mm diameter cellulose ester
plasticizers. Samples should be taken preferably in metal cans
surfactant-free membrane filters with a nominal pore size of
previously cleaned according to Practice D4057. Borosilicate
0.8 µm.
glass containers can be used if they are wrapped or boxed to
7.7.1 Single filters are to be used for prefiltration.
exclude light. Do not use soft (soda) glass containers.)
7.7.2 A matched weight pair of filters or alternatively, a
9.3 Test Samples—Reduction of the laboratory sample to
preweighed control and sample, filters shall be used for
test sample size (about 400 mL for each determination)
determination of filterable insolubles
depends upon the size of sample received by the laboratory. If
7.8 Evaporating Vessel, borosilicate glass beaker, 200-mL
the laboratory sample is stored in a tank, drum, or 19-L(5-gal)
capacity, tall style.
or larger can, use the pertinent procedures of Practice D4057.
7.9 Hot Plate, capable of heating a liquid in the evaporating
Thoroughly mix smaller laboratory samples by shaking, roll-
vessel (7.8) to 135°C (275°F).
ing, or other techniques before taking an aliquot portion by
8. Reagents and Materials
pouring, pipetting, or other means. Clean any tube, thief, pipet,
beaker, or other substance that is to contact the laboratory
8.1 Purity of Reagents—Reagent grade chemicals shall be
sample with trisolvent and rinse with a portion of the sample
used in all tests. Unless otherwise indicated, it is intended that
prior to use. Prior to mixing thoroughly and taking an aliquot,
all reagents shall conform to the specifications of the Commit-
tee onAnalytical Reagents of theAmerican Chemical Society,
Reagent Chemicals, American Chemical Society Specifications, American
This apparatus is available from suppliers of specialty petroleum testing Chemical Society, Washington, DC. For Suggestions on the testing of reagents not
equipment. listed by the American Chemical Society, see Annual Standards for Laboratory
Supporting data have been filed at ASTM International Headquarters and may Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
be obtained by requesting Research Report D02-1012. Filters may be qualified and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
using the procedure in this research report. MD.
D2274–03a (2008)
bath is extended to 40 h. Periods other than 16 h may also be used in
allow samples that have been stored at temperatures much
research or in other specifications. However, the precision values given in
below 10°C (50°F) to warm to room temperature; thus allow-
Test Method D2274 apply only to the 16 h period in the heating bath.
ing any separated wax to redissolve and to allow the viscosity
to decrease to a point where mixing is effective.
11.3 Cooling the Sample:
11.3.1 Sixteen 60.25hfromzerotime,removethesamples
10. Preparation of Apparatus
fromtheheatingbathinthesamesequenceastheywereplaced
therein. Cover the opening of each cell with a piece of
10.1 Preparation of Glassware Other Than Oxidation
aluminum foil or plastic to prevent entrance of dirt, dust, or
Cells—Rinse all glassware thoroughly with trisolvent followed
moisture. Record the time the first cell is removed.
bywater,thenwashwithamildlyalkalineorneutrallaboratory
11.3.2 Place in a dark, ventilated site at room temperature,
detergent. Rinse three times with deionized or distilled water
which shall be above the cloud point of the fuel, until the fuel
followed by acetone to remove water.
attains room temperature but for no longer than 4 h.
10.2 Preparation of Oxidation Cells and Accessories—
11.4 Determining Filterable Insolubles:
After completion of 10.1, fill oxidation cells with laboratory
11.4.1 Assemble the filter apparatus as illustrated in Fig. 2
detergent in water. Place the oxygen delivery tube in the
using one set of matched pair filters.Apply suction or vacuum
oxidation cell, place the condenser over the oxygen delivery
as necessary to filter the sample in an adequate time (approxi-
tube and allow to soak at least two hours. Wash, drain, then
mately 80 kPa (12 psi)); pour the cooled sample through the
rinse five times w
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
An American National Standard
Designation:D2274–03a Designation:D2274–03a (Reapproved 2008)
Designation:388/97
Standard Test Method for
Oxidation Stability of Distillate Fuel Oil (Accelerated
Method)
This standard is issued under the fixed designation D 2274; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope
1.1 This test method covers the measurement of the inherent stability of middle distillate petroleum fuels under specified
oxidizing conditions at 95°C.
NOTE 1—Fuels used in establishing the precision measures for this test method were described as gas oil, diesel fuel, No. 2 heating oil, and DFM, a
Navy distillate fuel suitable for diesels, boilers, and gas turbines. (The term DFM is no longer used when referring to fuel meeting MIL-F-16884
requirements; rather it is called F76 as it conforms to NATO F76 requirements.) While the test method may be used for fuels outside the range of these
fuels, the precision measures may not apply.
1.2 This test method is not applicable to fuels containing residual oil or significant amounts of components derived from
non-petroleum sources.
1.3 The values given in acceptable SI units are to be regarded as the standard. The values in parentheses are for information
only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
D 381 Test Method for Gum Content in Fuels by Jet Evaporation
D 943 Test Method for Oxidation Characteristics of Inhibited Mineral Oils
D 1193 Specification for Reagent Water
D 4057 Practice for Manual Sampling of Petroleum and Petroleum Products
D 4177 Practice for Automatic Sampling of Petroleum and Petroleum Products
D 4625 Test Method for Distillate Fuel Storage Stability at 43°C (110°F) Test Method for Middle Distillate Fuel Storage
Stability at 43C (110F)
2.2 Military Specification:
MIL-F-16884 Fuel, Navy Distillate
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 adherent insolubles (formerly adherent gum)—material which is produced in the course of stressing distillate fuel under
the conditions of this test and which adheres to the glassware after fuel has been flushed from the system.
3.1.2 filterable insolubles—material, which is produced in the course of stressing distillate fuel under the conditions of this test,
which is capable of being removed from the fuel by filtration.This includes both material suspended in the fuel and material easily
This test method is under the jurisdiction of Committee D02 on Petroleum Products and Lubricants and is the direct responsibility of Subcommittee D02.14 on Stability
and Cleanliness of Liquid Fuels.
Current edition approved Nov.Dec. 1, 2003.2008. Published December 2003.February 2009. Originally approved in 1964. Last previous edition approved in 2003 as
D 2274–03a.
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Available from Standardization Documents Order Desk, Bldg. 4, 700 Robbins Ave., Philadelphia, PA 19111-5098. Attn: NPODS
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D2274–03a (2008)
removed from the oxidation cell and oxygen delivery tube with hydrocarbon solvent.
3.1.3 inherent stability—the resistance to change when exposed to air, but in the absence of other environmental factors such
as water, or reactive metallic surfaces and dirt.
3.1.4 total insolubles—sum of the adherent and filterable insolubles.
3.1.5 zero time—the time the first of a batch of oxidation cells is placed in the heating bath.
3.1.5.1 Discussion—This is the time taken as the start of the 16 h of residence in the heating bath.
4. Summary of Test Method
4.1 A 350-mL volume of filtered middle distillate fuel is aged at 95°C (203°F) for 16 h while oxygen is bubbled through the
sample at a rate of 3 L/h. After aging, the sample is cooled to approximately room temperature before filtering to obtain the
filterable insolubles quantity. Adherent insolubles are then removed from the oxidation cell and associated glassware with
trisolvent. The trisolvent is evaporated to obtain the quantity of adherent insolubles. The sum of the filterable and adherent
insolubles, expressed as milligrams per 100 mL, is reported as total insolubles.
5. Significance and Use
5.1 This test method provides a basis for the estimation of the storage stability of middle distillate fuels such as No. 2 fuel oil.
5.2 The test method may not provide a prediction of the quantity of insolubles that will form in field storage over any given
period of time. The amount of insolubles formed in such field storage is subject to the specific conditions which are too variable
for this test method to predict accurately.
5.3 Test Method D 2274 yields results more rapidly than Test Method D 4625, the 43°C bottle test. However, as a result of the
significantly elevated temperature and the pure oxygen atmosphere, the nature and amount of insolubles may deviate to a greater
extent than Test Method D 4625 from those formed in field storage.
6. Interferences
6.1 Oxidation is a major chemical process causing adherent and filterable insolubles to form.Any substance such as copper or
chromium that catalyzes oxidation reactions will cause greater quantities of insolubles to form. Since the apparatus used in this
test can also be used in Test Method D 943, where coils of copper and steel are used, it is important that any residues that could
contain these metals be eliminated from the apparatus by thorough cleaning prior to use. Similarly, to preclude the presence of
chromium ions, as well as to protect laboratory personnel from potential harm, chromic acid shall not be used for cleaning
glassware in the practice of this method.
6.2 Ithasbeenfoundthatcommercialgradesofacetone,ifusedinthetrisolvent,canhaveimpuritieswhichcauseanapparently
greater level of adherent insolubles to be measured. It is, therefore, critical that only reagent (or higher) grade materials be used
in preparing the trisolvent mixture.
6.3 Ultraviolet light exposure has been found to increase the amount of total insolubles. Therefore, the fuel being tested shall
be shielded from direct exposure to ultraviolet light (sunlight or fluorescent). Conduct all sampling, measuring, filtration, and
weighing away from direct sunlight and in as dark an area as would be compatible with other laboratory operations. Storage before
stress, the stress period and cool-down after stressing shall be in the dark.
7. Apparatus
NOTE 2—It is suggested that all equipment be calibrated according to manufacturer’s instructions on a periodic basis to assure consistency of results.
7.1 Oxidation Cell, of borosilicate glass, as shown in Fig. 1, shall consist of a test tube, condenser, and oxygen delivery tube.
This cell is identical to that used in Test Method D 943.
7.2 Heating Bath, with a thermostatically controlled liquid medium, shall be capable of maintaining the bath temperature at 95
6 0.2°C (203 6 0.4°F). It shall be fitted with a suitable stirring device to provide a uniform temperature throughout the bath. It
shall be large enough to hold the desired number of oxidation cells immersed to a depth of approximately 350 mm. Further, the
bath construction must permit shielding the fuel samples in the oxidation cells from light while they are undergoing oxidation.
7.3 Flowmeters,shallhaveacapabilityofmeasuring3 60.3L/hofoxygen.Oneflowmetershallbeprovidedforeachoxidation
cell.
7.4 Filter Drying Oven, shall be capable of safely evaporating the solvent at 80° 6 2°C (176° 6 4°F) for the drying of filter
materials.
7.5 Glassware Drying Oven, shall be capable of drying glassware at 105° 6 5°C (221 6 9°F).
7.6 Filter Assembly, see Fig. 2, shall be capable of holding the filters described in 7.7.
7.7 Filter Media , 47 mm diameter cellulose ester surfactant-free membrane filters with a nominal pore size of 0.8 µm.
7.7.1 Single filters are to be used for prefiltration.
This apparatus is available from suppliers of specialty petroleum testing equipment.
Supporting data have been filed atASTM International Headquarters and may be obtained by requesting Research Report RR: D02-1012. Filters may be qualified using
the procedure in this research report.
D2274–03a (2008)
FIG. 1 Oxidation Cell
FIG. 2 Apparatus for Determining Filterable Insolubles
7.7.2 Amatched weight pair of filters or alternatively, a preweighed control and sample, filters shall be used for determination
of filterable insolubles
7.8 Evaporating Vessel, borosilicate glass beaker, 200-mL capacity, tall style.
7.9 Hot Plate, capable of heating a liquid in the evaporating vessel (7.8) to 135°C (275°F).
8. Reagents and Materials
8.1 Purity of Reagents—Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all
reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where
D2274–03a (2008)
such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high
purity to permit its use without lessening the accuracy of the determination.
8.2 Purity of Water— Unless otherwise indicated, reference to water shall be understood to mean reagent water as defined by
Type III of Specification D 1193.
8.3 2.2,4-trimethylpentanel (isooctane) , 99.75 % purity prefiltered through a filter medium of the type specified in 7.7.
8.4 Oxygen, 99.5 % purity or better. When the oxygen is delivered through a plant system of piping, a filter shall be provided
adjacent to the constant temperature bath to prevent the introduction of line debris or moisture into the oxidation cells; a pressure
regulator adequate to maintain a constant flow of gas through the apparatus shall also be used. A tank of oxygen of the specified
purity can be used provided it is equipped with a two-stage pressure regulator. ( Warning—Oxygen vigorously accelerates
combustion. Do not use equipment having exposed surfaces containing oil or grease.)
8.5 Trisolvent, a mixture of equal volumes of acetone, methanol, and toluene. See 8.1. (Warning—It is particularly important
that technical, commercial, practical, or industrial grades (however they are designated by the particular manufacturer) are not to
be used, as their use may lead to apparently increased levels of adherent insolubles.) ( Warning—Fire hazard, toxic.)
9. Samples and Sampling
9.1 When obtaining samples for the laboratory, follow Practices D 4057 or D 4177, or other standard practice capable of
providing representative samples.
9.2 Analyze fuel samples as soon as possible after receipt. When a fuel cannot be tested within one day, blanket it with an inert
gas such as oxygen-free nitrogen, argon, or helium and store at a temperature no higher than 10°C (50°F) but not lower than the
cloud point. (Warning—Plastic containers are not acceptable for samples due to the potential for leaching of plasticizers. Samples
shouldbetakenpreferablyinmetalcanspreviouslycleanedaccordingtoPracticeD 4057.Borosilicateglasscontainerscanbeused
if they are wrapped or boxed to exclude light. Do not use soft (soda) glass containers.)
9.3 Test Samples—Reductionofthelaboratorysampletotestsamplesize(about400mLforeachdetermination)dependsupon
the size of sample received by the laboratory. If the laboratory sample is stored in a tank, drum, or 19-L (5-gal) or larger can, use
the pertinent procedures of Practice D 4057. Thoroughly mix smaller laboratory samples by shaking, rolling, or other techniques
before taking an aliquot portion by pouring, pipetting, or other means. Clean any tube, thief, pipet, beaker, or other substance that
is to contact the laboratory sample with trisolvent and rinse with a portion of the sample prior to use. Prior to mixing thoroughly
and taking an aliquot, allow samples that have been stored at temperatures much below 10°C (50°F) to warm to room temperature;
thus allowing any separated wax to redissolve and to allow the viscosity to decrease to a point where mixing is effective.
10. Preparation of Apparatus
10.1 Preparation of Glassware Other Than Oxidation Cells—Rinse all glassware thoroughly with trisolvent followed by water,
then wash with a mildly alkaline or neutral laboratory detergent. Rinse three times with deionized or distilled water followed by
acetone to remove water.
10.2 Preparation of Oxidation Cells and Accessories —After completion of 10.1, fill oxidation cells with laboratory detergent
in water. Place the oxygen delivery tube in the oxidation cell, place the condenser over the oxygen delivery tube and allow to soak
at least two hours. Wash, drain, then rinse five times with tap water followed by three rinses with distilled or deionized water
meeting Specification D 1193 Type III requirements. Rinse with acetone; drain and allow the oxidation cell and oxygen delivery
tube to dry.
10.3 Preparation of Evaporating Beakers— Dry the 200-mL cleaned beakers (10.1) for1hinan oven at 105° 6 5°C (221 6
9°F). Place the beakers in a desiccator (without desiccant) and allow to cool for 1 h. Weigh beakers to the nearest 0.1 mg.
11. Procedure
11.1 Preparing the Sample—Place one filter (described in 7.7) on the filter support and clamp the filter funnel to the support
as shown in Fig. 2.Apply suction (approximately 80 kPa (12 psi)). Pour 400 mLof the fuel through the filter (see 7.7) into a clean
(10.1) 500-mL glass suction flask. Repeat preparation for each sample to be run. After filtration is complete, discard the filter
media. Never use the same filters for a second increment of fuel, because any material deposited on the filters by a previous
increme
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
An American National Standard
Designation:D2274–03 Designation:D2274–03a (Reapproved 2008)
Designation:388/97
Standard Test Method for
Oxidation Stability of Distillate Fuel Oil (Accelerated
Method)
This standard is issued under the fixed designation D 2274; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope
1.1 This test method covers the measurement of the inherent stability of middle distillate petroleum fuels under specified
oxidizing conditions at 95°C.
NOTE 1—Fuels used in establishing the precision measures for this test method were described as gas oil, diesel fuel, No. 2 heating oil, and DFM, a
Navy distillate fuel suitable for diesels, boilers, and gas turbines. (The term DFM is no longer used when referring to fuel meeting MIL-F-16884
requirements; rather it is called F76 as it conforms to NATO F76 requirements.) While the test method may be used for fuels outside the range of these
fuels, the precision measures may not apply.
1.2 This test method is not applicable to fuels containing residual oil or significant amounts of components derived from
non-petroleum sources.
1.3 The values given in acceptable SI units are to be regarded as the standard. The values in parentheses are for information
only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
D 381 Test Method for Gum Content in Fuels by Jet Evaporation
D 943 Test Method for Oxidation Characteristics of Inhibited Mineral Oils
D 1193 Specification for Reagent Water
D 4057 Practice for Manual Sampling of Petroleum and Petroleum Products
D 4177 Practice for Automatic Sampling of Petroleum and Petroleum Products
D 4625 Test Method for Distillate Fuel Storage Stability at 43°C (110°F) Test Method for Middle Distillate Fuel Storage
Stability at 43C (110F)
2.2 Military Specification:
MIL-F-16884 Fuel, Navy Distillate
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 adherent insolubles (formerly adherent gum)—material which is produced in the course of stressing distillate fuel under
the conditions of this test and which adheres to the glassware after fuel has been flushed from the system.
3.1.2 filterable insolubles—material, which is produced in the course of stressing distillate fuel under the conditions of this test,
This test method is under the jurisdiction of Committee D02 on Petroleum Products and Lubricants and is the direct responsibility of Subcommittee D02.14 on Stability
and Cleanliness of Liquid Fuels.
Current edition approved June 10, 2003. Published July 2003. Originally approved in 1964. Last previous edition approved in 2001 as D2274–01a.
Current edition approved Dec. 1, 2008. Published February 2009. Originally approved in 1964. Last previous edition approved in 2003 as D 2274–03a.
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
, Vol 05.01.volume information, refer to the standard’s Document Summary page on the ASTM website.
Annual Book of ASTM Standards, Vol 11.01.
Available from Standardization Documents Order Desk, Bldg. 4, 700 Robbins Ave., Philadelphia, PA 19111-5098. Attn: NPODS
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D2274–03a (2008)
which is capable of being removed from the fuel by filtration.This includes both material suspended in the fuel and material easily
removed from the oxidation cell and oxygen delivery tube with hydrocarbon solvent.
3.1.3 inherent stability—the resistance to change when exposed to air, but in the absence of other environmental factors such
as water, or reactive metallic surfaces and dirt.
3.1.4 total insolubles—sum of the adherent and filterable insolubles.
3.1.5 zero time—the time the first of a batch of oxidation cells is placed in the heating bath.
3.1.5.1 Discussion—This is the time taken as the start of the 16 h of residence in the heating bath.
4. Summary of Test Method
4.1 A 350-mL volume of filtered middle distillate fuel is aged at 95°C (203°F) for 16 h while oxygen is bubbled through the
sample at a rate of 3 L/h. After aging, the sample is cooled to approximately room temperature before filtering to obtain the
filterable insolubles quantity. Adherent insolubles are then removed from the oxidation cell and associated glassware with
trisolvent. The trisolvent is evaporated to obtain the quantity of adherent insolubles. The sum of the filterable and adherent
insolubles, expressed as milligrams per 100 mL, is reported as total insolubles.
5. Significance and Use
5.1 This test method provides a basis for the estimation of the storage stability of middle distillate fuels such as No. 2 fuel oil.
5.2 The test method may not provide a prediction of the quantity of insolubles that will form in field storage over any given
period of time. The amount of insolubles formed in such field storage is subject to the specific conditions which are too variable
for this test method to predict accurately.
5.3 Test Method D 2274 yields results more rapidly than Test Method D 4625, the 43°C bottle test. However, as a result of the
significantly elevated temperature and the pure oxygen atmosphere, the nature and amount of insolubles may deviate to a greater
extent than Test Method D 4625 from those formed in field storage.
6. Interferences
6.1 Oxidation is a major chemical process causing adherent and filterable insolubles to form.Any substance such as copper or
chromium that catalyzes oxidation reactions will cause greater quantities of insolubles to form. Since the apparatus used in this
test can also be used in Test Method D 943, where coils of copper and steel are used, it is important that any residues that could
contain these metals be eliminated from the apparatus by thorough cleaning prior to use. Similarly, to preclude the presence of
chromium ions, as well as to protect laboratory personnel from potential harm, chromic acid shall not be used for cleaning
glassware in the practice of this method.
6.2 Ithasbeenfoundthatcommercialgradesofacetone,ifusedinthetrisolvent,canhaveimpuritieswhichcauseanapparently
greater level of adherent insolubles to be measured. It is, therefore, critical that only reagent (or higher) grade materials be used
in preparing the trisolvent mixture.
6.3 Ultraviolet light exposure has been found to increase the amount of total insolubles. Therefore, the fuel being tested shall
be shielded from direct exposure to ultraviolet light (sunlight or fluorescent). Conduct all sampling, measuring, filtration, and
weighing away from direct sunlight and in as dark an area as would be compatible with other laboratory operations. Storage before
stress, the stress period and cool-down after stressing shall be in the dark.
7. Apparatus
NOTE 2—It is suggested that all equipment be calibrated according to manufacturer’s instructions on a periodic basis to assure consistency of results.
7.1 Oxidation Cell, of borosilicate glass, as shown in Fig. 1, shall consist of a test tube, condenser, and oxygen delivery tube.
This cell is identical to that used in Test Method D 943.
7.2 Heating Bath, with a thermostatically controlled liquid medium, shall be capable of maintaining the bath temperature at 95
6 0.2°C (203 6 0.4°F). It shall be fitted with a suitable stirring device to provide a uniform temperature throughout the bath. It
shall be large enough to hold the desired number of oxidation cells immersed to a depth of approximately 350 mm. Further, the
bath construction must permit shielding the fuel samples in the oxidation cells from light while they are undergoing oxidation.
7.3 Flowmeters,shallhaveacapabilityofmeasuring3 60.3L/hofoxygen.Oneflowmetershallbeprovidedforeachoxidation
cell.
7.4 Filter Drying Oven, shall be capable of safely evaporating the solvent at 80° 6 2°C (176° 6 4°F) for the drying of filter
materials.
7.5 Glassware Drying Oven, shall be capable of drying glassware at 105° 6 5°C (221 6 9°F).
7.6 Filter Assembly, see Fig. 2, shall be capable of holding the filters described in 7.7.
7.7 Filter Media , 47 mm diameter cellulose ester surfactant-free membrane filters with a nominal pore size of 0.8 µm.
Annual Book of ASTM Standards, Vol 05.02.
This apparatus is available from suppliers of specialty petroleum testing equipment.
Available from Standardization Documents Order Desk, Bldg. 4, 700 Robbins Ave., Philadelphia, PA 19111-5098. Attn: NPODS
D2274–03a (2008)
FIG. 1 Oxidation Cell
FIG. 2 Apparatus for Determining Filterable Insolubles
7.7.1 Single filters are to be used for prefiltration.
7.7.2 Amatched weight pair of filters or alternatively, a preweighed control and sample, filters shall be used for determination
of filterable insolubles
7.8 Evaporating Vessel, borosilicate glass beaker, 200-mL capacity, tall style.
7.9 Hot Plate, capable of heating a liquid in the evaporating vessel (7.8) to 135°C (275°F).
8. Reagents and Materials
8.1 Purity of Reagents—Reagent grade chemicals shall be used in all tests. Unless otherwise indicated, it is intended that all
reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical Society, where
Supporting data have been filed atASTM International Headquarters and may be obtained by requesting Research Report RR: D02-1012. Filters may be qualified using
the procedure in this research report.
D2274–03a (2008)
such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of sufficiently high
purity to permit its use without lessening the accuracy of the determination.
8.2 Purity of Water— Unless otherwise indicated, reference to water shall be understood to mean reagent water as defined by
Type III of Specification D 1193.
8.3 2.2,4-trimethylpentanel (Isooctane) 2.2,4-trimethylpentanel (isooctane) , 99.75 % purity prefiltered through a filter medium
of the type specified in 7.7.
8.4 Oxygen, 99.5 % purity or better. When the oxygen is delivered through a plant system of piping, a filter shall be provided
adjacent to the constant temperature bath to prevent the introduction of line debris or moisture into the oxidation cells; a pressure
regulator adequate to maintain a constant flow of gas through the apparatus shall also be used. A tank of oxygen of the specified
purity can be used provided it is equipped with a two-stage pressure regulator. ( Warning—Oxygen vigorously accelerates
combustion. Do not use equipment having exposed surfaces containing oil or grease.)
8.5 Trisolvent, a mixture of equal volumes of acetone, methanol, and toluene. See 8.1. (Warning—It is particularly important
that technical, commercial, practical, or industrial grades (however they are designated by the particular manufacturer) are not to
be used, as their use may lead to apparently increased levels of adherent insolubles.) ( Warning—Fire hazard, toxic.)
9. Samples and Sampling
9.1 When obtaining samples for the laboratory, follow Practices D 4057 or D 4177, or other standard practice capable of
providing representative samples.
9.2 Analyze fuel samples as soon as possible after receipt. When a fuel cannot be tested within one day, blanket it with an inert
gas such as oxygen-free nitrogen, argon, or helium and store at a temperature no higher than 10°C (50°F) but not lower than the
cloudpoint(seeAppendixX1).point.(Warning—Plasticcontainersarenotacceptableforsamplesduetothepotentialforleaching
of plasticizers. Samples should be taken preferably in metal cans previously cleaned according to Practice D 4057. Borosilicate
glass containers can be used if they are wrapped or boxed to exclude light. Do not use soft (soda) glass containers.)
9.3 Test Samples—Reductionofthelaboratorysampletotestsamplesize(about400mLforeachdetermination)dependsupon
the size of sample received by the laboratory. If the laboratory sample is stored in a tank, drum, or 19-L (5-gal) or larger can, use
the pertinent procedures of Practice D 4057. Thoroughly mix smaller laboratory samples by shaking, rolling, or other techniques
before taking an aliquot portion by pouring, pipetting, or other means. Clean any tube, thief, pipet, beaker, or other substance that
is to contact the laboratory sample with trisolvent and rinse with a portion of the sample prior to use. Prior to mixing thoroughly
and taking an aliquot, allow samples that have been stored at temperatures much below 10°C (50°F) to warm to room temperature;
thus allowing any separated wax to redissolve and to allow the viscosity to decrease to a point where mixing is effective.
10. Preparation of Apparatus
10.1 Preparation of Glassware Other Than Oxidation Cells—Rinse all glassware thoroughly with trisolvent followed by water,
then wash with a mildly alkaline or neutral laboratory detergent. Rinse three times with deionized or distilled water followed by
acetone to remove water.
10.2 Preparation of Oxidation Cells and Accessories —After completion of 10.1, fill oxidation cells with laboratory detergent
in water. Place the oxygen delivery tube in the oxidation cell, place the condenser over the oxygen delivery tube and allow to soak
at least two hours. Wash, drain, then rinse five times with tap water followed by three rinses with distilled or deionized water
meeting Specification D 1193 Type III requirements. Rinse with acetone; drain and allow the oxidation cell and oxygen delivery
tube to dry.
10.3 Preparation of Evaporating Beakers— Dry the 200-mL cleaned beakers (10.1) for1hinan oven at 105° 6 5°C (221 6
9°F). Place the beakers in a desiccator (without desiccant) and allow to cool for 1 h. Weigh beakers to the nearest 0.1 mg.
11. Procedure
11.1 Preparing the Sample—Place one filter (described in 7.7) on the filter support and clamp the filter funne
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.