ASTM F2313-03
(Specification)Standard Specification for Virgin Poly(glycolide) and Poly(glycolide-co-lactide) Resins for Surgical Implants with Mole Fractions Greater Than or Equal to 70% Glycolide
Standard Specification for Virgin Poly(glycolide) and Poly(glycolide-co-lactide) Resins for Surgical Implants with Mole Fractions Greater Than or Equal to 70% Glycolide
SCOPE
1.1 This specification covers both virgin poly(glycolide) resin and poly(glycolide-co-lactide) resin with mole fractions greater than or equal to 70 % glycolide. This specification is not applicable to glycolide:lactide copolymers with mole fractions exceeding 30 % lactide.
1.2 Since poly(glycolide) is commonly abbreviated as PGA for poly(glycolic acid) and poly(lactide) is commonly abbreviated as PLA for poly(lactic acid), these polymers are commonly referred to as PGA and PGA:PLA resins for the hydrolytic byproducts to which they respectively degrade.
1.3 This specification addresses material characteristics of both virgin poly(glycolide) and poly(≥70 % glycolide-co-lactide) resins intended for use in surgical implants and does not apply to packaged and sterilized finished implants fabricated from this material.
1.4 As with any material, some characteristics may be altered by processing techniques (such as molding, extrusion, machining, assembly, sterilization, and so forth) required for the production of a specific part or device. Therefore, properties of fabricated forms of this resin should be evaluated independently using appropriate test methods to ensure safety and efficacy.
1.5 This standard may suggest use of hazardous materials, operations, and equipment. This standard does not purport to address safety concerns associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and to determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F 2313 – 03
Standard Specification for
Virgin Poly(glycolide) and Poly(glycolide-co-lactide) Resins
for Surgical Implants with Mole Fractions Greater Than or
Equal to 70 % Glycolide
This standard is issued under the fixed designation F 2313; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope D 1898 Practice for Sampling of Plastics
D 2857 Practice for Dilute Solution Viscosity of Polymers
1.1 This specification covers both virgin poly(glycolide)
D 3536 Test Method for Molecular Weight Averages and
resin and poly(glycolide-co-lactide) resin with mole fractions
Molecular Weight Distribution by Liquid Exclusion Chro-
greater than or equal to 70 % glycolide. This specification is
matography (Gel Permeation Chromatography—GPC)
not applicable to glycolide:lactide copolymers with mole
D 3593 Test Method for Molecular Weight Averages and
fractions exceeding 30 % lactide.
Molecular Weight Distribution of Certain Polymers by
1.2 Since poly(glycolide) is commonly abbreviated as PGA
Liquid Size-Exclusion Chromatography (Gel Permeation
for poly(glycolic acid) and poly(lactide) is commonly abbre-
Chromatography—GPC) Using Universal Calibration
viated as PLA for poly(lactic acid), these polymers are com-
D 4603 Test Method for Determining Inherent Viscosity of
monly referred to as PGA and PGA:PLA resins for the
Poly(Ethylene Terephthalate) (PET) by Glass Capillary
hydrolytic byproducts to which they respectively degrade.
Viscometer
1.3 This specification addresses material characteristics of
E 386 Practice for Data Presentation Relating to High-
both virgin poly(glycolide) and poly($70 % glycolide-co-
Resolution Nuclear Magnetic Resonance (NMR) Spectros-
lactide) resins intended for use in surgical implants and does
copy
not apply to packaged and sterilized finished implants fabri-
E 1252 Practice for General Techniques for Obtaining In-
cated from this material.
frared Spectra for Qualitative Analysis
1.4 As with any material, some characteristics may be
F 748 Practice for Selecting Generic Biological Test Meth-
altered by processing techniques (such as molding, extrusion,
ods for Materials and Devices
machining, assembly, sterilization, and so forth) required for
2.2 Other Standards:
the production of a specific part or device. Therefore, proper-
United States Pharmacopeia (USP) Edition 26
ties of fabricated forms of this resin should be evaluated
ISO 10993-9 Biological Evaluation of Medical Devices,
independently using appropriate test methods to ensure safety
Part 9: Framework for Identification and Quantification of
and efficacy.
Potential Degradation Products, Annex A
1.5 This standard may suggest use of hazardous materials,
21 CFR 820, United States Code of Federal Regulations,
operations, and equipment. This standard does not purport to
Title 21—Food and Drugs Services, Part 820—Quality
address safety concerns associated with its use. It is the
System Regulation
responsibility of the user of this standard to establish appro-
ANSI/ISO/ASQ Q9000-2000, Quality Management Sys-
priate safety and health practices and to determine the
tems; Fundamentals and Vocabulary
applicability of regulatory limitations prior to use.
ANSI/ISO/ASQ Q9001-2000, Quality Management Sys-
2. Referenced Documents tems; Requirements
2.1 ASTM Standards:
3. Terminology
D 1505 Test Method for Density of Plastics by the Density-
3.1 Definitions:
Gradient Technique
This specification is under the jurisdiction of ASTM Committee F04 on
Medical Surgical Materials and Devices and is the direct responsibility of Subcom- Withdrawn.
mittee F04.11 on Polymeric Materials. Available from U.S. Pharmacopeia (USP), 12601Twinbrook Pkwy., Rockville,
Current edition approved Nov. 1, 2003. Published November 2003. MD 20852.
2 5
For referenced ASTM standards, visit the ASTM website, www.astm.org, or Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
contactASTM Customer Service at service@astm.org. ForAnnual Book ofASTM 4th Floor, New York, NY 10036.
Standards volume information, refer to the standard’s Document Summary page on AvailablefromU.S.GovernmentPrintingOfficeSuperintendentofDocuments,
the ASTM website. 732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F2313–03
3.1.1 virgin polymer, n—the form of poly(glycolide) or 5.2.4.2 Additional spectral bands may be indicative of
poly(glycolide-co-lactide) as synthesized from its monomers known or unknown impurities, including residual solvents and
and prior to fabrication into a medical device. catalysts (refer to limits specified in Table 1).
5.3 Molecular Weight:
4. Materials and Manufacture
5.3.1 The molecular mass of the virgin polymer shall be
indicated by inherent viscosity in dilute solution (IV). In
4.1 All raw monomer components and other materials
addition to inherent viscosity (but not in place of), weight
contacting either the raw monomer(s) or resin product shall be
average molecular mass and molecular mass distributions may
of a quality suitable to allow for use of such resin in the
be determined by gel permeation chromatography (GPC)
manufacture of an implantable medical product.
according to Test Methods D 3536 or D 3593, but using
4.2 All polymer manufacturing (including monomer han-
hexafluoroisopropanol (HFIP) solvent and poly methyl-
dling, synthesis, pelletization/grinding and all subsequent)
methacrylate (PMMA) calibration standards.
shall be undertaken under conditions suitable to allow for use
of such resin in the manufacture of an implantable medical 5.3.1.1 Determine the inherent viscosity of the polymer
product. either in hexafluoroisopropanol (HFIP) or hexafluoroacetone
sesquihydrate (HFAS) at 30°C using procedures similar to
those described in Practice D 2857 and Test Method D 4603.
5. Chemical Composition
Inherent viscosity is determined utilizing the following equa-
5.1 Polymers covered by this specification shall be com-
tion:
posed either of glycolide, or of a combination of glycolide and
lactidewherethelactidecontentdoesnotexceed30 %(34.7 % t
ln ~v!
t
by weight). To ensure such composition and the attainment of o
IV 5 (1)
w
the desired properties, the following tests are to be conducted.
5.2 Chemical Identification:
where:
5.2.1 The identity of the virgin polymer shall be confirmed
IV = inherent viscosity (at 30°C in dl/gram),
1 13
either by infrared, H-NMR, or C-NMR spectroscopy.
t = efflux time in seconds for diluted solution,
5.2.2 Infrared Identification:
t = efflux time in seconds for source solvent,
o
5.2.2.1 Identity of either poly(glycolide) homopolymer or
w = weight of polymer being diluted (in grams), and
poly(glycolide-co-lactide) copolymer may be confirmed
v = dilution volume in deciliters (Note: 1 dl = 100 mL).
through an infrared spectrum exhibiting major absorption
Resin concentration for IV analysis must be 0.5 % w/v or
bands only at the wavelengths that appear in a suitable
less, with resin analyte concentrations of 0.1 % w/v (that is,
reference spectrum. Analysis shall be conducted using prac-
0.001 g/ml or 1 mg/ml) recommended.When reporting results,
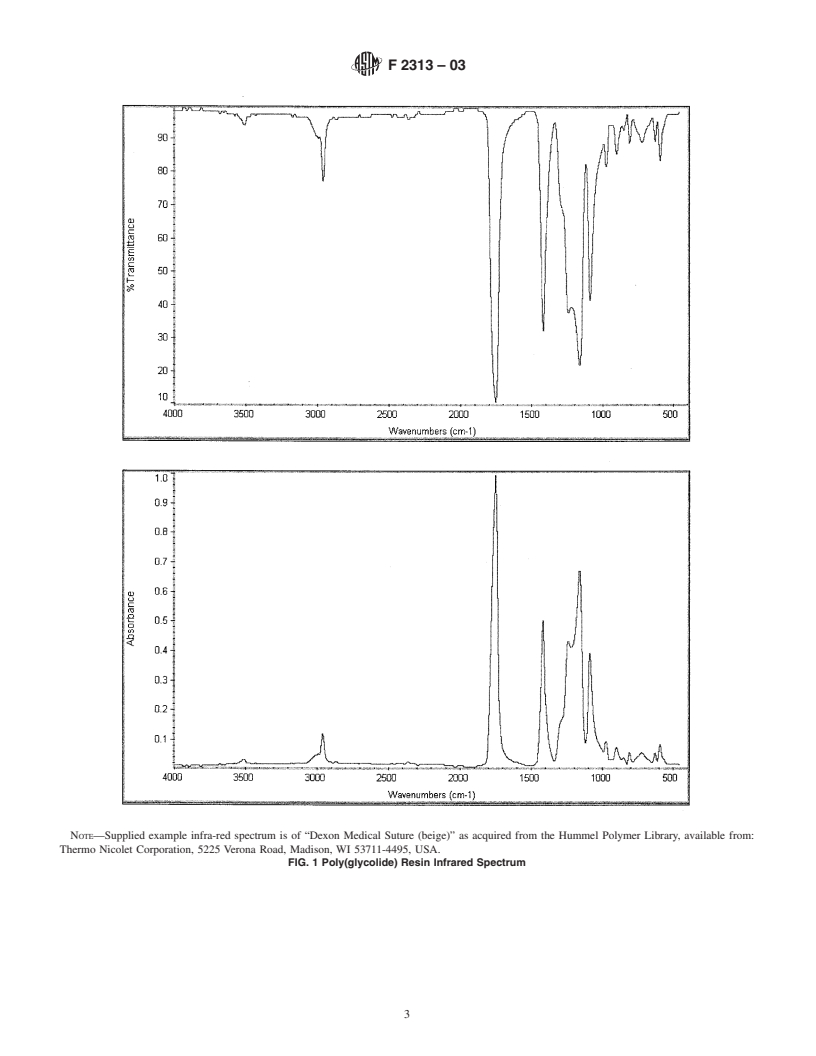
tices similar to those described in Practice E 1252. A typical
identify the solvent utilized, analyte concentration, and analy-
infrared transmission reference spectrum for PGA homopoly-
sis temperature.
mer is shown in Fig. 1. A typical infrared transmission
5.4 Residual Monomer:
reference spectrum for a 90 % PGA:10 % l-PLAcopolymer is
5.4.1 The virgin polymer shall have a combined total
shown in Fig. 2.
residual monomer content less than or equal to 2 % by weight.
5.2.2.2 Additional spectral bands may be indicative of
5.4.1.1 Determine weight percent residual monomer by gas
known or unknown impurities, including residual solvents and
chromatography, H-NMR spectroscopy (using D-HFIP or
catalysts (refer to limits specified in Table 1).
otherproton-freesolventabletofullysolvatethespecimen),or
5.2.3 Proton Nuclear Magnetic Resonance ( H-NMR) Iden-
other suitably sensitive analytic method as agreed upon by
tification:
supplier and purchaser.
5.2.3.1 Identity of either poly(glycolide) homopolymer or
5.5 Residual Solvents:
poly(glycolide-co-lactide) copolymer may be confirmed
5.5.1 If any solvent is utilized in any resin manufacturing or
through sample dissolution, H-NMR spectroscopy, and the use
purification step, determine residual levels of any utilized
of a suitable reference spectrum. Sample dissolution is in
solvent(s) by gas chromatography or other suitable method as
deuterated hexafluoroisopropanol (D-HFIP) or other proton-
agreed upon by supplier and purchaser. Acceptable residual
free solvent able to fully solvate the specimen. Analysis shall
levels of a solvent shall be reflective of toxicity, with a
be conducted using practices similar to those described in
maximum acceptable level (regardless of toxicity) presented in
Practice E 386.
Table 1.
5.2.3.2 Additional spectral bands may be indicative of
5.6 Heavy Metals:
known or unknown impurities, including residual solvents and
5.6.1 Determine residual Heavy Metals per Method II,
catalysts (refer to limits specified in Table 1).
Chapter 231 of U.S. Pharmacopeia.
5.2.4 Carbon-13 Nuclear Magnetic Resonance ( C-NMR)
Identification:
5.6.2 Heavy Metals generally refers to divalent cations of
5.2.4.1 Identity of either poly(glycolide) homopolymer or the elements antimony (Sb), arsenic (As), cadmium (Cd),
poly(glycolide-co-lactide) copolymer may be confirmed in a copper (Cu), mercury (Hg), and lead (Pb). Since stannous tin
13 2+
solid state through C-NMR spectroscopy and the use of a (Sn ) carries potential to influence test results, the amount
suitable reference spectrum.Analysis shall be conducted using ascertained by alternative analytic means (see below) to be
practices similar to those described in Practice E 386. directly attributable to tin in may be ignored, provided that the
F2313–03
NOTE—Supplied example infra-red spectrum is of “Dexon Medical Suture (beige)” as acquired from the Hummel Polymer Library, available from:
Thermo Nicolet Corporation, 5225 Verona Road, Madison, WI 53711-4495, USA.
FIG. 1 Poly(glycolide) Resin Infrared Spectrum
F2313–03
NOTE—Supplied example infra-red spectrum is of “Vicryl Medical Suture (violet)” as acquired from the Hummel Polymer Library, available from:
Thermo Nicolet Corporation, 5225 Verona Road, Madison, WI 53711-4495, USA.
FIG. 2 Poly(90 % glycolide-co-10 % lactide) Resin Infrared Spectrum
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.