ASTM F3368-19
(Guide)Standard Guide for Cell Potency Assays for Cell Therapy and Tissue Engineered Products
Standard Guide for Cell Potency Assays for Cell Therapy and Tissue Engineered Products
SIGNIFICANCE AND USE
5.1 Cell Therapy Products may be used to treat clinical conditions, for example in regenerative medicine (e.g. type I diabetes, acute myocardial infarction, pediatric congenital heart disease, chronic ischemic heart failure, cancer, Crohn’s disease, chronic wound repair, nerve and spinal cord injury, musculoskeletal repair), and may be used for immunotherapy (e.g. graft versus host disease, CAR-T therapy).
5.2 Autologous, allogeneic, and xenogeneic cells may be used to make a product.
5.3 A product may be cells only, cells combined with an inert carrier, cells within an extracellular matrix, or cells within a synthetic scaffold, and will include tissue engineered medical products containing cells.
5.4 Cells may be gene-modified cells.
5.5 Cells may be adult or embryonic stem cells.
5.6 Cells may be minimally manipulated.
SCOPE
1.1 This guide is intended as a resource for individuals and organizations involved in the development, production, delivery, and regulation of cellular therapy products (CTPs) including genetically modified cells, tissue engineered medical products (TEMPs) and combination products where cell activity is a functional component of the final product.
1.2 This Guide was developed to include input derived from several previously published guidance documents and standards (section 2.4). It is the intent of this Guide is to reflect the current perspectives for CTP potency assays.
1.3 CTPs can provide therapy by localized or systemic treatment of a disease or pathology.
1.4 The products may provide a relatively short therapy, may be transient, or may be permanent and provide long-term therapy.
1.5 The products may be cells alone, cells combined with a carrier that is transient, or cells combined with a scaffold or other components that function in the overall therapy.
1.6 Potency assays may be in-vitro or in-vivo assays designed to determine the potency of a specific product. In-vivo assays are likely to be particularly useful to study the mechanism of action (MOA) of the therapy, but may not be desirable for final product quality control where they may be time-consuming and expensive, and where in-vitro assays may be preferable.
1.7 It is likely that multiple assays, and possibly both in-vitro and in-vivo assays, will be required to provide a broad measure of potency. However, in-vitro assays are likely to be preferred as release assays for products, and so studies to identify potency assays should emphasize in-vitro assays that are correlative or predictive of preclinical or clinical results.
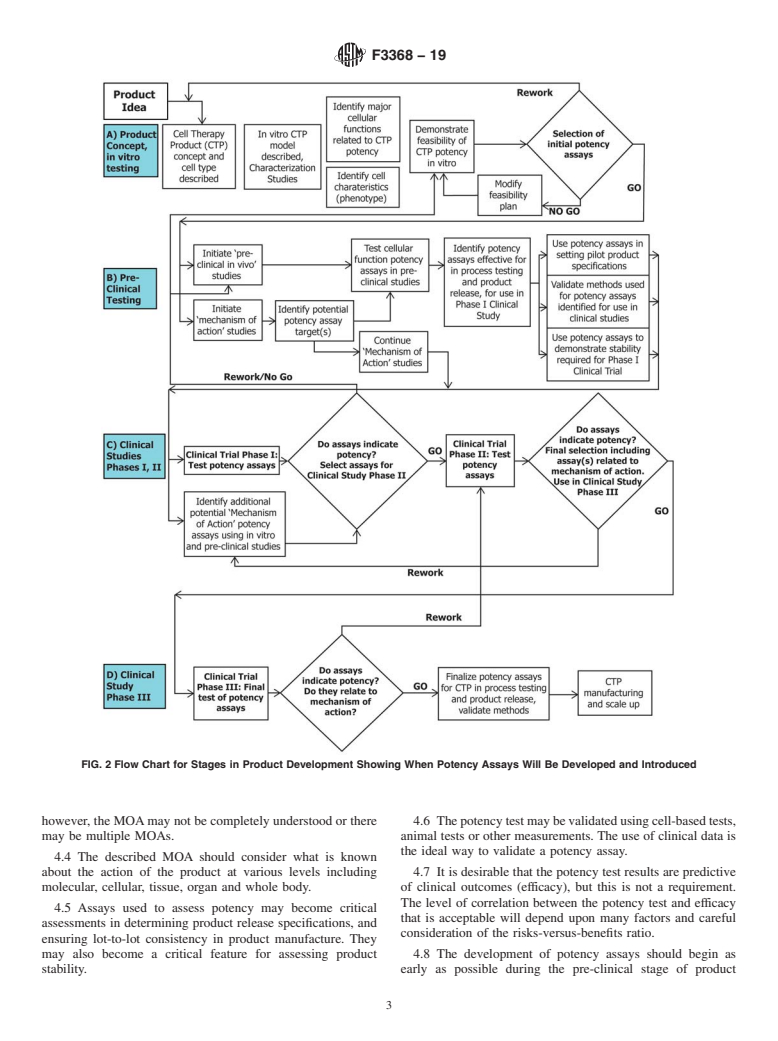
1.8 Potency assays should be developed during the product development cycle and therefore are likely to be more comprehensive at the end of that cycle compared to the beginning of product development and testing. It is recommended that potency assays be developed as early as possible in the product development cycle (Figs. 1 and 2).
FIG. 1 Progressive Implementation of Potency Assays
FIG. 2 Flow Chart for Stages in Product Development Showing When Potency Assays Will Be Developed and Introduced
1.9 Potency measurements are used as part of the testing for cell and cell-based products to demonstrate that product lots meet defined specifications when released for clinical use.
1.10 Shelf life specifications should be developed during the product development process to include potency measurements.
1.11 This standard guide is not intended to apply to drug or gene therapy products. However, genetically modified cell therapies, for example the chimeric antigen receptor-T (CAR-T) cell therapy, which the United States FDA classifies as gene therapy, are applicable.
1.12 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limit...
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F3368 − 19
Standard Guide for
Cell Potency Assays for Cell Therapy and Tissue
1
Engineered Products
This standard is issued under the fixed designation F3368; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope prehensive at the end of that cycle compared to the beginning
of product development and testing. It is recommended that
1.1 This guide is intended as a resource for individuals and
potency assays be developed as early as possible in the product
organizations involved in the development, production,
development cycle (Figs. 1 and 2).
delivery, and regulation of cellular therapy products (CTPs)
including genetically modified cells, tissue engineered medical 1.9 Potency measurements are used as part of the testing for
products (TEMPs) and combination products where cell activ- cell and cell-based products to demonstrate that product lots
ity is a functional component of the final product. meet defined specifications when released for clinical use.
1.2 This Guide was developed to include input derived from 1.10 Shelflifespecificationsshouldbedevelopedduringthe
several previously published guidance documents and stan- product development process to include potency measure-
dards (section 2.4). It is the intent of this Guide is to reflect the ments.
current perspectives for CTP potency assays.
1.11 This standard guide is not intended to apply to drug or
1.3 CTPs can provide therapy by localized or systemic gene therapy products. However, genetically modified cell
treatment of a disease or pathology. therapies, for example the chimeric antigen receptor-T
(CAR-T) cell therapy, which the United States FDA classifies
1.4 The products may provide a relatively short therapy,
as gene therapy, are applicable.
may be transient, or may be permanent and provide long-term
1.12 This standard does not purport to address all of the
therapy.
safety concerns, if any, associated with its use. It is the
1.5 The products may be cells alone, cells combined with a
responsibility of the user of this standard to establish appro-
carrier that is transient, or cells combined with a scaffold or
priate safety, health, and environmental practices and deter-
other components that function in the overall therapy.
mine the applicability of regulatory limitations prior to use.
1.6 Potency assays may be in-vitro or in-vivo assays de-
1.13 This international standard was developed in accor-
signed to determine the potency of a specific product. In-vivo
dance with internationally recognized principles on standard-
assays are likely to be particularly useful to study the mecha-
ization established in the Decision on Principles for the
nism of action (MOA) of the therapy, but may not be desirable
Development of International Standards, Guides and Recom-
for final product quality control where they may be time-
mendations issued by the World Trade Organization Technical
consuming and expensive, and where in-vitro assays may be
Barriers to Trade (TBT) Committee.
preferable.
2. Referenced Documents
1.7 It is likely that multiple assays, and possibly both
2
in-vitro and in-vivo assays, will be required to provide a broad
2.1 ASTM Standards:
measure of potency. However, in-vitro assays are likely to be
F2312 Terminology Relating to Tissue Engineered Medical
preferred as release assays for products, and so studies to
Products
identify potency assays should emphasize in-vitro assays that
3
2.2 ISO Document:
are correlative or predictive of preclinical or clinical results.
ISO 17025 General Requirements for the Competence of
1.8 Potency assays should be developed during the product
Testing and Calibration Laboratories
development cycle and therefore are likely to be more com-
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
1
This guide is under the jurisdiction of ASTM Committee F04 on Medical and contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Surgical Materials and Devices and is the direct responsibility of Subcommittee Standards volume information, refer to the standard’s Document Summary page on
F04.44 on Assessment for TEMPs. the ASTM website.
3
Current edition approved May 15, 2019. Published July 2019. DOI: 10.1520/ Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
F3368-19 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, W
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.