ASTM F1223-08(2012)
(Test Method)Standard Test Method for Determination of Total Knee Replacement Constraint
Standard Test Method for Determination of Total Knee Replacement Constraint
SIGNIFICANCE AND USE
4.1 This test method, when applied to available products and proposed prototypes, is meant to provide a database of product functionality capabilities (in light of the suggested test regimens) that is hoped will aid the physician in making a more informed total knee replacement (TKR) selection.
4.2 A proper matching of TKR functional restorative capabilities and the recipient's (patient's) needs is more likely to be provided by a rational testing protocol of the implant in an effort to reveal certain device characteristics pertinent to the selection process.
4.3 The TKR product designs are varied and offer a wide range of constraint (stability). The constraint of the TKR in the in vitro condition depends on several geometrical and kinematic interactions among the implant's components which can be identified and quantified. The degree of TKR's kinematic interactions should correspond to the recipient's needs as determined by the physician during clinical examination.
4.4 For mobile bearing knee systems, the constraint of the entire implant construct shall be characterized. Constraint of mobile bearings is dictated by design features at both the inferior and superior articulating interfaces.
4.5 The methodology, utility, and limitations of constraint/laxity testing are discussed.3, 4 The authors recognize that evaluating isolated implants (that is, without soft tissues) does not directly predict in vivo behavior, but will allow comparisons among designs. Constraint testing is also useful for characterizing implant performance at extreme ranges of motion which may be encountered in vivo at varying frequencies, depending on the patient’s anatomy, pre-operative capability, and post-operative activities and lifestyle.
SCOPE
1.1 This test method covers the establishment of a database of total knee replacement (TKR) motion characteristics with the intent of developing guidelines for the assignment of constraint criteria to TKR designs. (See the Rationale in Appendix X1.)
1.2 This test method covers the means by which a TKR constraint may be quantified according to motion delineated by the inherent articular design as determined under specific loading conditions in an in vitro environment.
1.3 Tests deemed applicable to the constraint determination are antero-posterior draw, medio-lateral shear, rotary laxity, valgus-varus rotation, and distraction, as applicable. Also covered is the identification of geometrical parameters of the contacting surfaces which would influence this motion and the means of reporting the test results. (See Practices E4.)
1.4 This test method is not a wear test.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F1223 −08(Reapproved 2012)
Standard Test Method for
Determination of Total Knee Replacement Constraint
This standard is issued under the fixed designation F1223; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope their human counterparts:
3.1.1 anterior curvature—a condylar design which is gen-
1.1 This test method covers the establishment of a database
erally planar except for a concave—upward region anteriorly
of total knee replacement (TKR) motion characteristics with
on the tibial component.
the intent of developing guidelines for the assignment of
constraint criteria to TKR designs. (See the Rationale in
3.1.2 anterior posterior (AP)—any geometrical length
Appendix X1.)
aligned with the AP orientation.
1.2 This test method covers the means by which a TKR
3.1.3 AP displacement—the relative linear translation be-
constraint may be quantified according to motion delineated by
tween components in the AP direction.
the inherent articular design as determined under specific
3.1.4 AP draw load—the force applied to the movable
loading conditions in an in vitro environment.
component with its vector aligned in the AP direction causing
1.3 Tests deemed applicable to the constraint determination
or intending to cause an AP displacement.
are antero-posterior draw, medio-lateral shear, rotary laxity,
3.1.5 biconcave—a condylar design with pronounced AP
valgus-varus rotation, and distraction, as applicable. Also
and MLcondylar radii seen as a “dish” in the tibial component
covered is the identification of geometrical parameters of the
or a “toroid” in the femoral component.
contacting surfaces which would influence this motion and the
3.1.6 bearing surface—those regions of the component
means of reporting the test results. (See Practices E4.)
which are intended to contact its counterpart for load transmis-
1.4 This test method is not a wear test.
sion.
1.5 The values stated in SI units are to be regarded as
3.1.7 condyles—entity designed to emulate the joint
standard. No other units of measurement are included in this
anatomy and used as a bearing surface primarily for transmis-
standard.
sion of the joint reaction force with geometrical properties
1.6 This standard does not purport to address all of the
which tend to govern the general kinematics of the TKR.
safety concerns, if any, associated with its use. It is the
3.1.8 distraction—the separation of the femoral compo-
responsibility of the user of this standard to establish appro-
nent(s) from the tibial component(s) in the z-direction.
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use. 3.1.9 femoral side constraint—that constraint provided by
the superior articulating interfaces, determined by fixing the
2. Referenced Documents
inferior surface of the mobile bearing component during
2.1 ASTM Standards:
testing.
E4 Practices for Force Verification of Testing Machines
3.1.10 flexion angle—the angulation of the femoral compo-
F2083 Specification for Total Knee Prosthesis
nent (about an axis parallel to the y-axis) from the fully
3. Terminology
extended knee position to a position in which a “local” vertical
axis on the component now points posteriorly.
3.1 Definitions—Items in this category refer to the geo-
3.1.10.1 Discussion—For many implants, 0° of flexion can
metricalandkinematicaspectsofTKRdesignsastheyrelateto
be defined as when the undersurface of the tibial component is
parallel to the femoral component surface that in vivo contacts
This test method is under the jurisdiction ofASTM Committee F04 on Medical
and Surgical Materials and Devices and is the direct responsibility of Subcommittee the most distal surface of the femur.This technique may not be
F04.22 on Arthroplasty.
possibleforsomeimplantsthataredesignedtohaveaposterior
Current edition approved Dec. 1, 2012. Published December 2012. Originally
tilt of the tibial component. In these cases, the user shall
approved in 1989. Last previous edition approved in 2008 as F1223 – 08. DOI:
specify how the 0° of flexion position was defined.
10.1520/F1223-08R12.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
3.1.11 hinge—a mechanical physical coupling between
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
femoral and tibial components which provides a single axis
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. about which flexion occurs.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F1223−08 (2012)
3.1.12 hyperextension stop—a geometrical feature which component.Theactualrelativemotionvaluesshallbeprovided
arrests further progress of flexion angles of negative value. as indicators of this type of constraint.
3.1.13 inferior articulating interfaces—any interface in 3.2.2 coordinate system (see Fig. 1)—a set of arbitrary
which relative motion occurs between the underside of the cartesian coordinates affixed to the stationary component and
mobile bearing component and the tibial tray. aligned such that the origin is located at the intersection of the
y and z axes.
3.1.14 internal-external rotation—the relative angulation of
3.2.2.1 Discussion—The y-axis is parallel to the ML
the moveable component about an axis parallel to the z-axis.
direction, directed medially, and is coincident with the mated
3.1.15 joint reaction force—theappliedloadwhosevectoris
components’ contact points when the knee is in the neutral
directed parallel to the z-axis, generally considered parallel to
position (see 7.2). The z-axis is located midway between the
tibial longitudinal axis.
mated components’ contact points (or in the case of a single
3.1.16 medio-lateral (ML)—the orientation that is aligned
contact point, located at that point) and aligned in the superior-
with the y-axis in the defined coordinate system.
inferior direction of the distal component. A third axis, x,
3.1.17 ML condylar radius—the geometrical curvature of mutually orthogonal to the two previous axes is directed
the component’s condyle in the frontal plane. posteriorly. For determination of contact points, see AnnexA1
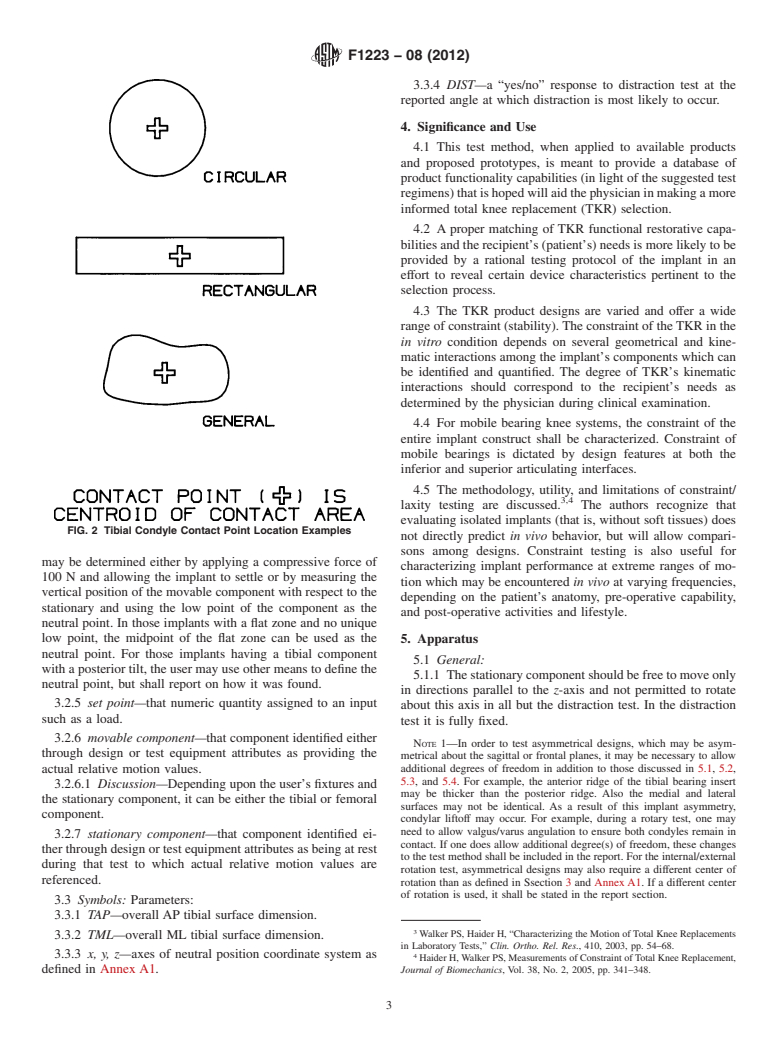
and Fig. 2. The contact point shall be located to a tolerance of
3.1.18 ML dimension—any geometrical length aligned with
61 mm. In the case of multiple contact points on a condyle, an
the ML orientation.
average location of the contact points shall be used.
3.1.19 ML displacement—the relative linear translation be-
3.2.3 degrees of freedom—although the knee joint is noted
tween components in the ML direction.
to have 6 df, or directions in which relative motion is guided
3.1.20 ML shear load—the force applied to the moveable
(three translations:AP, ML, vertical; three angulations: flexion,
component with its vector aligned in the ML direction and
internal-external rotation, valgus-varus), the coupling effects
causing or intending to cause an ML displacement.
due to geometrical features reduce this number to five which
3.1.21 mobile bearing component—the ultra-high molecular
are the bases of this test method: AP draw, ML shear,
weight polyethylene (UHMWPE) component that, by design,
internal-external rotation, valgus-varus rotation, and distrac-
articulates against both the femoral bearing and the tibial tray.
tion.
3.1.22 mobile bearing knee system—a knee prosthesis
3.2.4 neutral position (see 7.2)—that position in which the
system, comprised of a tibial component, a mobile bearing
TKR is at rest with no relative linear or angular displacements
component that can rotate or rotate and translate relative to the
between components.
tibial component, and a femoral component.
3.2.4.1 Discussion—This is design-dependent and there
may be a unique neutral position at each flexion angle. It may
3.1.23 post-in-well feature—a TKR design which tends to
be indicated that the femoral component, when implanted, be
influence kinematics through the coupling of a prominent
positioned at some angle of hyperextension as seen when the
eminence with a recess or housing in a mating component.
patient’s knee is fully extended; this, then becomes the neutral
3.1.24 rotary laxity(RL)—degreeofrelativeangularmotion
position for negative flexion angle tests. The neutral position
permitted for a moveable component about the z-axis as
governed by inherent geometry and load conditions.
3.1.25 rotary torque—the moment applied to the moveable
component with its vector aligned to an axis parallel to the
z-axis and causing or intending to cause an internal or external
rotation.
3.1.26 superior articulating interfaces—any interface in
whichrelativemotionoccursbetweenthetopsideofthemobile
bearing component and the femoral bearing component.
3.1.27 tibial eminence—a raised geometrical feature sepa-
rating the tibial condyles.
3.1.28 tibial side constraint—thatconstraintprovidedbythe
inferior articulating interface.
3.1.29 valgus-varus constraint—degree of relative angular
motion allowed between the femoral and tibial components of
post-in-well designs (or similar designs) in the coronal plane.
3.2 Definitions of Terms Specific to This Standard:
3.2.1 constraint—the relative inability of a TKR to be
further displaced in a specific direction under a given set of
loading conditions as dictated by the TKR’s geometrical
design. This motion is limited, as defined in this test, to the
available articular or bearing surfaces found on the tibial FIG. 1 Defined Coordinate System Examples
F1223−08 (2012)
3.3.4 DIST—a “yes/no” response to distraction test at the
reported angle at which distraction is most likely to occur.
4. Significance and Use
4.1 This test method, when applied to available products
and proposed prototypes, is meant to provide a database of
product functionality capabilities (in light of the suggested test
regimens)thatishopedwillaidthephysicianinmakingamore
informed total knee replacement (TKR) selection.
4.2 A proper matching of TKR functional restorative capa-
bilities and the recipient’s (patient’s) needs is more likely to be
provided by a rational testing protocol of the implant in an
effort to reveal certain device characteristics pertinent to the
selection process.
4.3 The TKR product designs are varied and offer a wide
range of constraint (stability).The constraint of theTKR in the
in vitro condition depends on several geometrical and kine-
matic interactions among the implant’s components which can
be identified and quantified. The degree of TKR’s kinematic
interactions should correspond to the recipient’s needs as
determined by the physician during clinical examination.
4.4 For mobile bearing knee systems, the constraint of the
entire implant construct shall be characterized. Constraint of
mobile bearings is dictated by design features at both the
inferior and superior articulating interfaces.
4.5 The methodology, utility, and limitations of constraint/
3,4
laxity testing are discussed. The authors recognize that
evaluating isolated implants (that is, without soft tissues) does
FIG. 2 Tibial Condyle Contact Point Location Examples
not directly predict in vivo behavior, but will allow compari-
sons among designs. Constraint testing is also useful for
may be determined either by applying a compressive force of
characterizing implant performance at extreme ranges of mo-
100 N and allowing the implant to settle or by measuring the
tion which may be encountered in vivo at varying frequencies,
vertical position of the movable component with respect to the
depending on the patient’s anatomy, pre-operative capability,
stationary and using the low point of the component as the
and post-operative activities and lifestyle.
neutral point. In those implants with a flat zone and no unique
low point, the midpoint of the flat zone can be used as the 5. Apparatus
neutral point. For those implants having a tibial component
5.1 General:
with a posterior tilt, the user may use other means to define the
5.1.1 Thestationarycomponentshouldbefreetomoveonly
neutral point, but shall report on how it was found.
in directions parallel to the z-axis and not permitted to rotate
3.2.5 set point—that numeric quantity assigned to an input
about this axis in all but the distraction test. In the distraction
such as a load.
test it is fully fixed.
3.2.6 movable component—that component identified either
NOTE 1—In order to test asymmetrical designs, which may be asym-
through design or test equipment attributes as providing the
metrical about the sagittal or frontal planes, it may be necessary to allow
additional degrees of freedom in addition to those discussed in 5.1, 5.2,
actual relative motion values.
5.3, and 5.4. For example, the anterior ridge of the tibial bearing insert
3.2.6.1 Discussion—Depending upon the user’s fixtures and
may be thicker than the posterior ridge. Also the medial and lateral
the stationary component, it can be either the tibial or femoral
surfaces may not be identical. As a result of this implant asymmetry,
component.
condylar liftoff may occur. For example, during a rotary test, one may
need to allow valgus/varus angulation to ensure both condyles remain in
3.2.7 stationary component—that component identified ei-
contact. If one does allow additional degree(s) of freedom, these changes
ther through design or test equipment attributes as being at rest
to the test method shall be included in the report. For the internal/external
during that test to which actual relative motion values are
rotation test, asymmetrical designs may also require a different center of
referenced.
rotation than as defined in Ssection 3 and Annex A1. If a different center
of rotation is used, it shall be stated in the report section.
3.3 Symbols: Parameters:
3.3.1 TAP—overall AP tibial surface dimension.
Walker PS, Haider H, “Characterizing the Motion of Total Knee Replacements
3.3.2 TML—overall ML tibial surface dimension.
in Laboratory Tests,” Clin. Ortho. Rel. Res., 410, 2003, pp. 54–68.
3.3.3 x, y, z—axes of neutral position coordinate system as
Haider H,Walker PS, Measurements of Constraint ofTotal Knee Replacement,
defined in Annex A1. Journal of Biomechanics, Vol. 38, No. 2, 2005, pp. 341–348.
F1223−08 (2012)
5.1.2 The movable component shall be the displaced mem- 5.5.1 The movable component shall be rigidly set in a
ber when under loads specific to that test and shall be fixture free to move in only t
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.