ASTM F746-87(1999)
(Test Method)Standard Test Method for Pitting or Crevice Corrosion of Metallic Surgical Implant Materials
Standard Test Method for Pitting or Crevice Corrosion of Metallic Surgical Implant Materials
SCOPE
1.1 This test method covers the determination of resistance to either pitting or crevice corrosion of metals and alloys from which surgical implants will be produced. It is a modified version of an established test and is used as a screening test to rank surgical implant alloys in order of their resistance to localized corrosion.
1.2 This test method applies only to passive metals and alloys. Nonpassive alloys (other than noble alloys) are susceptible to general corrosion and are not normally suitable for implant use.
1.3 The test method is intended for use as a laboratory screening test of metals and alloys which undergo pitting or crevice corrosion, or both.
1.4 The values stated in SI units are to be regarded as the standard.
1.5 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all of the safety problems associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F 746 – 87 (Reapproved 1999)
Standard Test Method for
Pitting or Crevice Corrosion of Metallic Surgical Implant
Materials
This standard is issued under the fixed designation F 746; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope a corrosion potential. Pitting (or crevice corrosion) is then
stimulated by potentiostatically polarizing the specimen to a
1.1 This test method covers the determination of resistance
potential much more noble than the corrosion potential. Stimu-
to either pitting or crevice corrosion of metals and alloys from
lation of pitting (or crevice corrosion) will be marked by a
which surgical implants will be produced. It is a modified
large and generally increasing polarizing current.
version of an established test and is used as a screening test to
3.2 Immediately after the stimulation step, the potential is
rank surgical implant alloys in order of their resistance to
decreased as rapidly as possible to one of several preselected
localized corrosion.
potentials at, or more noble than, the corrosion potential. If the
1.2 This test method applies only to passive metals and
alloy is susceptible to pitting (or crevice corrosion) at the
alloys. Nonpassive alloys (other than noble alloys) are suscep-
preselected potential, the polarizing current will remain at
tible to general corrosion and are not normally suitable for
relatively high values and will fluctuate or increase with time.
implant use.
A post-test examination of the metal specimen establishes
1.3 The test method is intended for use as a laboratory
whether localized corrosion has occurred by pitting of the
screening test of metals and alloys which undergo pitting or
exposed surface or by preferential attack at the crevice formed
crevice corrosion, or both.
by the tapered collar, or both.
1.4 The values stated in SI units are to be regarded as the
3.3 If the pit (or crevice) surface repassivates at the pre-
standard.
elected potential and localized corrosion is halted, the polariz-
1.5 This standard does not purport to address all of the
ing current will drop to values typical for passive surfaces and
safety concerns, if any, associated with its use. It is the
the current will decrease continuously. The parameter of
responsibility of the user of this standard to establish appro-
interest, the critical potential for pitting (or crevice corrosion),
priate safety and health practices and determine the applica-
is defined as the highest (most noble) preselected potential at
bility of regulatory limitations prior to use.
which pit (or crevice) surfaces repassivate after the stimulation
2. Referenced Documents step.
2.1 ASTM Standards:
4. Significance and Use
G 3 Practice for Conventions Applicable to Electrochemical
3 4.1 This method is designed solely for determining com-
Measurements in Corrosion Testing
parative laboratory indices of performance. The results may be
G 5 Standard Reference Test Method for Making Potentio-
used for ranking alloys in order of increasing resistance to
static and Potentiodynamic Anodic Polarization Measure-
pitting and crevice corrosion under the specific conditions of
ments
this method. It should be noted that the method is intentionally
G 15 Terminology Relating to Corrosion and Corrosion
designed to reach conditions that are sufficiently severe to
Testing
cause breakdown of at least one alloy (Type 316 L stainless
3. Summary of Test Method
steel) currently considered acceptable for surgical implant use,
and that those alloys which suffer pitting or crevice corrosion
3.1 A cylindrical specimen fitted with an inert tapered collar
during the more severe portions of the test do not necessarily
is immersed in a saline electrolyte at 37°C for1hto establish
suffer localized corrosion when placed within the human body
as a surgical implant.
This test method is under the jurisdiction of ASTM Committee F-4 on Medical
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
5. Apparatus
F04.15 on Material Test Methods.
Current edition approved March 27, 1987. Published May 1987. Originally 5.1 The following required equipment is described in Prac-
published as F 746 – 81. Last previous edition F 746 – 81.
tice G 5:
Syrett, B. C., Corrosion, Vol 33, 1977, p. 221.
5.1.1 Standard Polarization Cell, of 1000 cm .
Annual Book of ASTM Standards, Vol 03.02.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F 746 – 87 (1999)
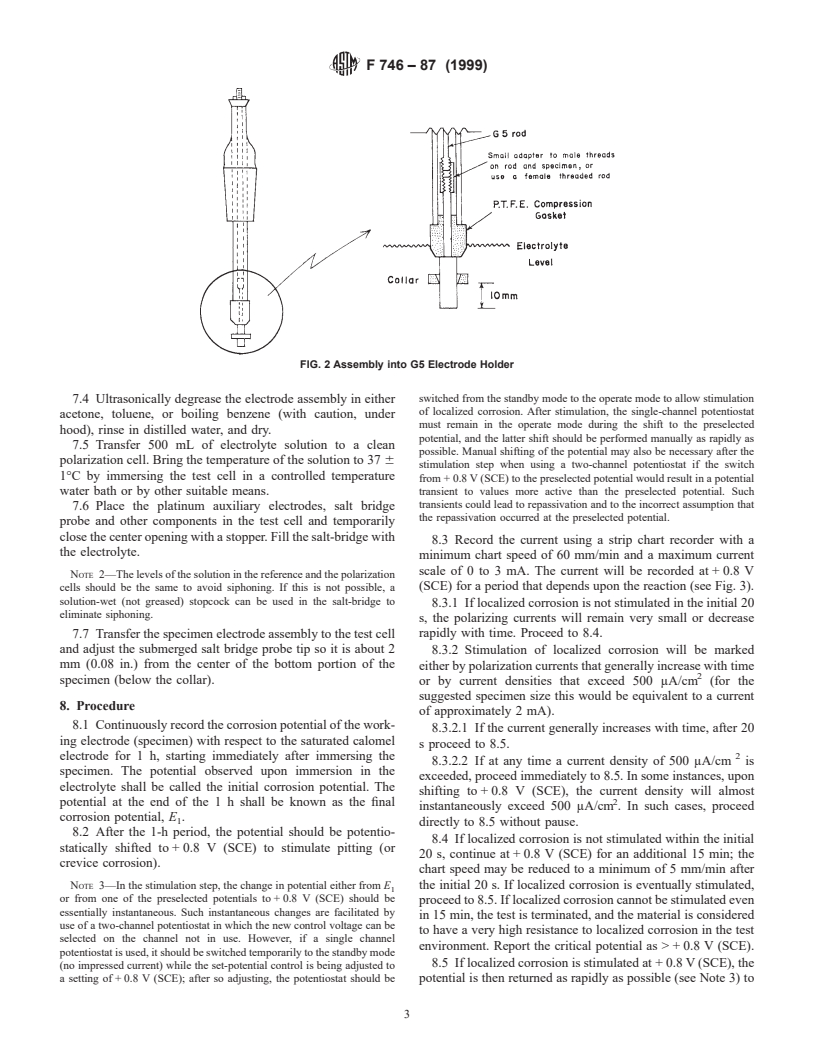
5.1.2 Electrode Holders, for auxiliary and working elec- 5.5 Determine the total exposed surface area of the speci-
trodes. men before placement of the PTFE collar, A ; determine the
T
area on the internal surface of the collar (the creviced area), A ;
5.1.3 Potentiostat, calibrated in accordance with Practice
C
G5. and determine the exposed surface area of the specimen after
placement of the collar, A (where: A = A − A ). Dimensions
5.1.4 Potential-Measuring Instrument.
S S T C
should be measured to the nearest 0.1 mm.
5.1.5 Current-Measuring Instrument.
5.5.1 Example—Using the dimensions suggested previously
5.1.6 Anodic Polarization Circuit.
for the specimen diameter ( d = 6.35 mm), the specimen length
5.1.7 Platinum Auxiliary Electrodes.
( l = 20.00 mm), and the collar thickness ( t = 3.18 mm),
5.1.8 Saturated Calomel Electrode (SCE).
5.1.9 Salt Bridge Probe.
pd
A 5pdl 1 5 431 mm (1)
T
5.2 A cylindrical working electrode is fabricated from the 4
test material by machining, grinding, and suggested final
polishing with 600-grit metallographic paper. It is suggested
A 5pdt 5 63 mm (2)
C
that the part of the cylindrical specimen that is exposed to the
A 5 A 2 A 5 386 mm (3)
S T C
test solution have a length of 20.00 6 1.00 mm (0.787 6 0.039
in.) and a diameter of 6.35 6 0.03 mm (0.250 6 0.001 in.) (see
Fig. 1).
6. Reagents
5.3 A crevice is created by fitting the cylindrical specimen
6.1 Electrolyte—Dissolve9gof reagent grade sodium
with a tapered collar, machined from commercial purity
chloride (NaCl) in distilled water to make 1000 cm of
polytetrafluoroethylene (PTFE). The collar should have an
solution.
outer diameter of 12.70 6 0.05 mm (0.500 6 0.002 in.) and a
6.1.1 After transferring the appropriate amount of electro-
thickness of 3.18 6 0.20 mm (0.1256 0.008 in.). The inside
lyte to the test cell (7.5), the pH is to be measured both before
diameter of the tapered collar should range from 0.38 mm
and after the test (see rationale in X1.6).
(0.015 in.) smaller than the diameter of the specimen to 0.38
mm (0.015 in.) larger. To be consistent with the dimensions
7. Preparation of Specimens and Conditioning
suggested in 5.2, the inside diameter should taper from 5.97 6
0.05 mm (0.235 6 0.002 in.) to 6.73 6 0.05 mm (0.265 6
7.1 Prepare the test specimen surface within1hofthe start
0.002 in.). See Fig. 1 for drawing of the tapered collar. The
of the experiment by the method described in Practice G 5.
relatively fine tolerances are needed to ensure a reproducible fit
7.2 Using a suitable mechanical jig, force-fit the PTFE
and crevice.
collar onto the cylindrical specimen so that the base of the
5.4 In Practice G 5, the method of specimen attachment is to
collar is up 10 6 2 mm (0.393 6 0.079 in.) from the bottom of
drill and tap the specimen to receive a threaded stainless steel
the specimen (see Fig. 2). Care should be taken to avoid
connection rod. A 4-40 thread is used, typically. However,
scratching the metal surface.
because many surgical implant alloys are not easily drilled,
NOTE 1—Once the collar is removed from the specimen, it should not
external threads may also be machined, ground, or cast, as
be reused.
illustrated in Fig. 1. A small stainless steel adapter is fitted on
to these threads and the adapter then accepts the connection
7.3 Mount the specimen on the holder and on the electrode
rod. rod as described in Practice G 5.
NOTE 1—Unless shown, dimensional tolerances are given in text.
FIG. 1 Dimensions of Specimen and Collar
F 746 – 87 (1999)
FIG. 2 Assembly into G5 Electrode Holder
switched from the standby mode to the operate mode to allow stimulation
7.4 Ultrasonically degrease the electrode assembly in either
of localized corrosion. After stimulation, the single-channel potentiostat
acetone, toluene, or boiling benzene (with caution, under
must remain in the operate mode during the shift to the preselected
hood), rinse in distilled water, and dry.
potential, and the latter shift should be performed manually as rapidly as
7.5 Transfer 500 mL of electrolyte solution to a clean
possible. Manual shifting of the potential may also be necessary after the
polarization cell. Bring the temperature of the solution to 37 6
stimulation step when using a two-channel potentiostat if the switch
1°C by immersing the test cell in a controlled temperature
from + 0.8 V (SCE) to the preselected potential would result in a potential
water bath or by other suitable means. transient to values more active than the preselected potential. Such
transients could lead to repassivation and to the incorrect assumption that
7.6 Place the platinum auxiliary electrodes, salt bridge
the repassivation occurred at the preselected potential.
probe and other components in the test cell and temporarily
close the center opening with a stopper. Fill the salt-bridge with
8.3 Record the current using a strip chart recorder with a
the electrolyte.
minimum chart speed of 60 mm/min and a maximum current
scale of 0 to 3 mA. The current will be recorded at + 0.8 V
NOTE 2—The levels of the solution in the reference and the polarization
(SCE) for a period that depends upon the reaction (see Fig. 3).
cells should be the same to avoid siphoning. If this is not possible, a
solution-wet (not greased) stopcock can be used in the salt-bridge to
8.3.1 If localized corrosion is not stimulated in the initial 20
eliminate siphoning.
s, the polarizing currents will remain very small or decrease
7.7 Transfer the specimen electrode assembly to the test cell rapidly with time. Proceed to 8.4.
and adjust the submerged salt bridge probe tip so it is about 2
8.3.2 Stimulation of localized corrosion will be marked
mm (0.08 in.) from the center of the bottom portion of the
either by polarization currents that generally increase with time
specimen (below the collar).
or by current densities that exceed 500 μA/cm (for the
suggested specimen size this would be equivalent to a current
8. Procedure
of approximately 2 mA).
8.1 Continuously record the corrosion potential of the work-
8.3.2.1 If the current generally increases with time, after 20
ing electrode (specimen) with respect to the saturated calomel
s proceed to 8.5.
electrode for 1 h, starting immediately after immersing the 2
8.3.2.2 If at any time a current density of 500 μA/cm is
specimen. The potential observed upon immersion in the
exceeded, proceed immediately to 8.5. In some instances, upon
electrolyte shall be called the initial corrosion potential. The
shifting to + 0.8 V (SCE), the current density will almost
potential at the end of the 1 h shall be known as the final
instantaneously exceed 500 μA/cm . In such cases, proceed
corrosion potential, E .
directly to 8.5 without pause.
8.2 After the 1-h period, the potential should be potentio-
8.4 If localized corrosion is not stimulated within the initial
statically shifted to + 0.8 V (SCE) to stimulate pitting (or
20 s, continue at + 0.8 V (SCE) for an additional 15 min; the
crevice corrosion).
chart speed may be reduced to a minimum of 5 mm/min after
NOTE 3—In the stimulation step, the change in potential either from E the initial 20 s. If localized corrosion is eventually stimulated,
or from one of the preselected potentials to + 0.8 V (SCE) should be
proceed to 8.5. If localized corrosion cannot be stimulated even
essentially instantaneous. Such instantaneous changes are facilitated by
in 15 min, the test is terminated, and the material is considered
use of a two-channel potentiostat in which the new control voltage can be
to have a very high resistance to localized corrosion in the test
selected on the channel not in use. However, if a single channel
environment. Report the critical potential as > + 0.8 V (SCE).
potentiostat is used, it should be switched temporarily to the standby mode
8.5 If localized corrosion is stimulated at + 0.8 V (SCE), the
(no impressed current) while the set-potential control is being adjusted to
a setting of + 0.8 V (SCE); after so adjusting, the potentiostat should be potential is then returned as rapidly as possible (see Note 3) to
F 746 – 87 (1999)
Note a—Current density instantly exceeds 500 μA/cm . Return immediately to preselected potential.
Note b—Current generally increases with time but does not ever exceed 500 μA/cm . Return to preselected potential after 20 s.
Note c—Localized corrosion is not stimulated within the initial 20 s. Continue for an additional 15 min.
Note d—If localized corrosion is eventually stimulated, return to preselected potential.
Note e—If localized corrosion cannot be stimulated even after the 15 min, the test is terminated.
FIG. 3 Stimulation of Localized Corrosion
E (which is the first preselected potential) to determine if the 8.6.1 During this 15 min, the chart speed may be reduced to
specimen will repassivate or if localized corrosion will con- a minimum of 5 mm/min.
tinue to propagate at the preselected potential.
8.6.2 Adjust the current scale to obtain satisfactory accu-
8.6 If the pitted or creviced local regions repassivate at the
racy. The range used for monitoring the relatively large current
preselected potential, the polarizing current will drop quickly
during stimulation is almost certainly unsuitable for accurately
to zero or to low values consistent with a passive surface
monitoring the much smaller repassivation currents.
condition (see Fig. 4(a) for examples). Monitor this current for
8.6.3 If the pitted or local regions do not repassivate at E ,
15 min.
then the critical voltage shall be reported as E , with the
notation that the specimen never repassivated following the
initial stimulation. The test shall be terminated.
8.7 After ensuring repassivation at E by observing low,
decreasing (or constant) polarization currents for 15 min,
repeat the stimulation step (8.2 and 8.3) at + 0.8 V (SCE) and
then change the potential as rapidly as possi
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.