ASTM G91-97(2004)
(Practice)Standard Practice for Monitoring Atmospheric SO2 Using the Sulfation Plate Technique
Standard Practice for Monitoring Atmospheric SO<inf>2</inf> Using the Sulfation Plate Technique
SIGNIFICANCE AND USE
Atmospheric corrosion of metallic materials is a function of many weather and atmospheric variables. The effect of specific corrodants, such as sulfur dioxide, can accelerate the atmospheric corrosion of metals significantly. The sulfation plate method provides a simple technique to independently monitor the level of SO2 in the atmosphere to yield a weighted average result.
Sulfation plate results may be used to characterize atmospheric corrosion test sites regarding the effective average level of SO2 in the atmosphere at these locations.
Sulfation plate testing is useful in determining microclimate, seasonal, and long term variations in the effective average level of SO2.
The results of sulfation plate tests may be used in correlations of atmospheric corrosion rates with atmospheric data to determine the sensitivity of the corrosion rate to SO2 level.
The sulfation plate method may also be used with other methods to characterize the atmosphere at sites where buildings or other construction is planned in order to determine the extent of protective measures required for metallic materials.
SCOPE
1.1 This practice covers a weighted average effective SO2 level for a 30-day interval through the use of the sulfation plate method, a technique for estimating the effective SO2 content of the atmosphere, and especially with regard to the atmospheric corrosion of stationary structures or panels. This practice is aimed at determining SO2 levels rather than sulfuric acid aerosol or acid precipitation.
1.2 The results of this practice correlate approximately with volumetric SO2 concentrations, although the presence of dew or condensed moisture tends to enhance the capture of SO2 into the plate.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:G91–97 (Reapproved 2004)
Standard Practice for
Monitoring Atmospheric SO Using the Sulfation Plate
Technique
This standard is issued under the fixed designation G91; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope arerecoveredandsulfateanalysesperformedonthecontentsto
determine the extent of sulfur capture. The results are reported
1.1 This practice covers a weighted average effective SO
in terms of milligrams of SO per square metre per day.
level for a 30-day interval through the use of the sulfation plate
method, a technique for estimating the effective SO content of
4. Significance and Use
the atmosphere, and especially with regard to the atmospheric
4.1 Atmospheric corrosion of metallic materials is a func-
corrosion of stationary structures or panels. This practice is
tion of many weather and atmospheric variables. The effect of
aimed at determining SO levels rather than sulfuric acid
specific corrodants, such as sulfur dioxide, can accelerate the
aerosol or acid precipitation.
atmospheric corrosion of metals significantly. The sulfation
1.2 The results of this practice correlate approximately with
plate method provides a simple technique to independently
volumetric SO concentrations, although the presence of dew
monitor the level of SO in the atmosphere to yield a weighted
orcondensedmoisturetendstoenhancethecaptureofSO into
average result.
the plate.
4.2 Sulfation plate results may be used to characterize
1.3 This standard does not purport to address all of the
atmospheric corrosion test sites regarding the effective average
safety concerns, if any, associated with its use. It is the
level of SO in the atmosphere at these locations.
responsibility of the user of this standard to establish appro-
4.3 Sulfation plate testing is useful in determining micro-
priate safety and health practices and determine the applica-
climate, seasonal, and long term variations in the effective
bility of regulatory limitations prior to use.
average level of SO .
2. Referenced Documents 4.4 The results of sulfation plate tests may be used in
correlations of atmospheric corrosion rates with atmospheric
2.1 ASTM Standards:
data to determine the sensitivity of the corrosion rate to SO
D516 Test Method for Sulfate Ion in Water
level.
D2010/D2010M Test Methods for Evaluation of Total Sul-
4.5 The sulfation plate method may also be used with other
fation Activity in the Atmosphere by the Lead Dioxide
methods to characterize the atmosphere at sites where build-
Technique
ings or other construction is planned in order to determine the
G16 Guide forApplying Statistics toAnalysis of Corrosion
extent of protective measures required for metallic materials.
Data
5. Interferences
3. Summary of Practice
5.1 The lead peroxide reagent used in this practice may
3.1 Sulfation plates consisting of a lead peroxide reagent in
convert other compounds such as mercaptans, hydrogen sul-
an inverted dish are exposed for 30-day intervals. The plates
fide, and carbonyl sulfide into sulfate.
NOTE 1—Hydrogen sulfide and mercaptans, at concentrations which
This practice is under the jurisdiction of ASTM Committee G01 on Corrosion
affect the corrosion of structural metals significantly, are relatively rare in
of Metals and is the direct responsibility of Subcommittee G01.04 on Atmospheric
most atmospheric environments, but their effects regarding the corrosion
Corrosion.
of metals are not equivalent to sulfur dioxide. Therefore, if H S, COS, or
Current edition approved Nov 1, 2004. Published November 2004. Originally
approved in 1986. Last previous edition approved on 1997 as G91 – 97. DOI: mercaptans are present in the atmosphere, the lead peroxide method must
10.1520/G0091-97R04.
not be used to assess atmospheric corrosivity. It should also be noted that
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
no actual measurements have been made which would establish the
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
correlation between atmospheric H S, COS, or mercaptan level and
Standards volume information, refer to the standard’s Document Summary page on
sulfation as measured by this practice.
the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
G91–97 (2004)
5.2 The inverted exposure position of the sulfation plate is recorded when the plate exposure is initiated. At the termina-
intended to minimize capture of sulfuric acid aerosols and tion of exposure, the completion date should be added to the
sulfur bearing species from precipitation. exposure records.
NOTE 3—The 30 day exposure is not very discriminating in areas of
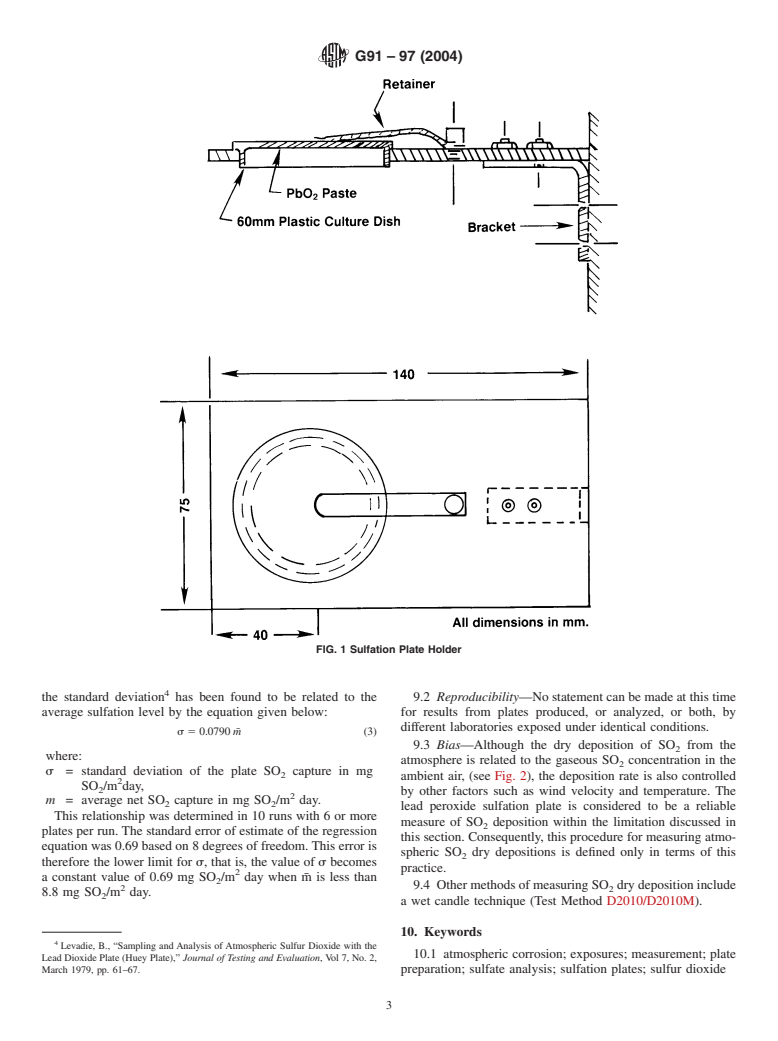
6. Sulfation Plate Preparation and Exposure
low SO concentrations. Experience has shown that 60- to 90-day
exposure may be necessary to develop a measurable SO capture on the
6.1 Sulfation plates can be prepared according to the 2
3 plate.
method of Huey. The plate preparation method is given in
Appendix X1. Laboratory prepared plates should be exposed 6.6 The sulfation plates shall be analyzed for sulfate content
within 120 days of preparation. using any established quantitative analysis technique.
6.2 Ingeneral,thelevelofatmosphericsulfurdioxidevaries
NOTE 4—In conducting the sulfate analysis, it is necessary to remove
seasonally during the year so that a minimal exposure program
the contents of the sulfation plate and solubilize the sulfate, for example,
requires four 30-day exposures each year at roughly equal
using a solution of sodium carbonate. It has been found that 20 mL of 50
intervals. In order to establish the atmospheric SO level at an g/L Na CO (ACS reagent grade) is sufficient to solubilize the sulfate in
2 2 3
this test method in a 3-hour period. Thereafter, conventional sulfate
atmospheric corrosion test site which has not been monitored
analysis can be employed, for example, by barium precipitation and either
previously, a program in which six 30-day exposures per year
gravimetric or turbidimetric analysis (see Test Methods D516).
for a period of 3 years is recommended. More extensive testing
may be desirable if large variability is encountered in the
7. Calculation
results. Thereafter, the location should be monitored with at
7.1 The sulfate analysis provides the quantity of sulfate on
least four tests in a 1-year period every 3 years. If the
eachdiscanalyzed.ThisshouldbeconvertedtoanSO capture
subsequent tests are not consistent with the initial testing, then
rate, R, by the following equation.
another 3-year program of six tests per year is required. Also,
R 5 ~m 2 mo! 3 MWSO /MWSO 3 A 3 T (1)
if a major change in the general area occurs in terms of 2 4
industrial or urban development, then six tests per year for 3
where:
years should again be carried out.
m = mass of sulfate found in the plate, mg,
6.3 In monitoring exposure sites, a minimum of four plates
m = mass of sulfate found in a blank (unexposed)
shall be used for each exposure period.
plate, mg,
6.3.1 Sites which have a grade or elevation variation should
MWSO = 64,
be monitored with at least two plates at the highest elevation
MWSO = 96,
and two plates at the lowest elevation.
A = area of the plate, m , and
T = exposure time of the plate, days.
6.3.2 Plates should be exposed, if possible, at both the
highest and lowest level above the ground at which corrosion
test specimens are exposed. 2
R 5 SO capture rate, mg SO /m day (2)
2 2
6.3.3 Sites larger than 10 000 m shall have at least eight
7.2 The SO capture rate may be converted to equivalent
plates exposed for each period. In rectangular sites on level
SO or SO values if desired, but for comparison purposes,
3 4
ground, it is desirable to expose two plates at each corner.
SO rates shall b
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.