ASTM F2392-04(2010)
(Test Method)Standard Test Method for Burst Strength of Surgical Sealants
Standard Test Method for Burst Strength of Surgical Sealants
SIGNIFICANCE AND USE
Materials and devices that function at least in part by adhering to living tissues are finding increasing use in surgical procedures, either as adjuncts to sutures and staples or as frank replacements for those devices in a wide variety of medical procedures. While the nature and magnitude of the forces involved varies greatly with indication and with patient specific circumstances, all uses involve, to some extent, the ability of the material to resist imposed mechanical forces. Therefore, the mechanical properties of the materials, and in particular the adhesive and cohesive properties, are important parameters in evaluating their fitness for use. In addition, the mechanical properties of a given sealant composition can provide a useful means of determining product consistency for quality control, or as a means for determining the effects of various surface treatments on the substrate prior to use of the device.
The complexity and variety of individual applications for sealant, even within a single indicated use (surgical procedure), is such that the results of a burst test are not suitable for determining allowable design stresses without thorough analysis and understanding of the application and sealant behaviors.
This test method may be used for comparing sealants for susceptibility to environmental changes, but such comparisons must be made with great caution since different sealants may respond differently to varying conditions.
As the true sealant strength is strongly dependent on the strength of the sealant/substrate interface, the selection of a proper test substrate is critical. Care must be taken when extrapolating in vitro test results to in vivo expectations. In vitro sealant optimization may not translate to expected in vivo performance due to differences in substrate surface, strength, and elasticity.
SCOPE
1.1 This test method provides a means for comparison of the burst or rupture strength of sealants on soft tissue. This test method can be used as a clinically relevant model for quality assurance, development, and comparative testing of different adhesives or adherends.
1.2 This test method measures only burst strength or “cohesive strength” of an adhesive/adherend system, and not the adhesive strength.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2392 − 04(Reapproved 2010)
Standard Test Method for
Burst Strength of Surgical Sealants
This standard is issued under the fixed designation F2392; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.2.2 burst strength—the average pressure required to cause
failure of the sealant, either by cohesive or adhesive mecha-
1.1 Thistestmethodprovidesameansforcomparisonofthe
nisms.
burst or rupture strength of sealants on soft tissue. This test
3.2.3 cohesive failure—failure of the sealant during burst
method can be used as a clinically relevant model for quality
testing.
assurance, development, and comparative testing of different
adhesives or adherends.
3.2.4 cohesive strength—the internal strength of the sealant,
sometimes referred to as the adhesive bulk strength.
1.2 This test method measures only burst strength or “co-
hesive strength” of an adhesive/adherend system, and not the
3.2.5 substrate failure—failure of the substrate during burst
adhesive strength.
testing.
1.3 The values stated in SI units are to be regarded as 3.2.6 tissue sealant—a surface coating to prevent leakage of
standard. No other units of measurement are included in this body fluids.
standard.
4. Significance and Use
1.4 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the 4.1 Materials and devices that function at least in part by
adhering to living tissues are finding increasing use in surgical
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- procedures, either as adjuncts to sutures and staples or as frank
replacements for those devices in a wide variety of medical
bility of regulatory limitations prior to use.
procedures. While the nature and magnitude of the forces
2. Referenced Documents
involvedvariesgreatlywithindicationandwithpatientspecific
circumstances, all uses involve, to some extent, the ability of
2.1 ASTM Standards:
thematerialtoresistimposedmechanicalforces.Therefore,the
D907 Terminology of Adhesives
mechanical properties of the materials, and in particular the
2.2 American Association for Tissue Banks (AATB) Stan-
adhesive and cohesive properties, are important parameters in
dard:
evaluating their fitness for use. In addition, the mechanical
Standards for Tissue Banking
properties of a given sealant composition can provide a useful
3. Terminology means of determining product consistency for quality control,
or as a means for determining the effects of various surface
3.1 Definitions—Many terms in this test method are defined
treatments on the substrate prior to use of the device.
in Terminology D907.
4.2 The complexity and variety of individual applications
3.2 Definitions:
for sealant, even within a single indicated use (surgical
3.2.1 adhesive failure—failure of the sealant/substrate inter-
procedure), is such that the results of a burst test are not
face during burst testing.
suitable for determining allowable design stresses without
thorough analysis and understanding of the application and
sealant behaviors.
This test method is under the jurisdiction ofASTM Committee F04 on Medical
and Surgical Materials and Devicesand is the direct responsibility of Subcommittee
4.3 Thistestmethodmaybeusedforcomparingsealantsfor
F04.15 on Material Test Methods.
susceptibility to environmental changes, but such comparisons
Current edition approved Sept. 1, 2010. Published November 2010. Originally
approved in 2004. Last previous edition approved in 2004 as F2392 – 04. DOI:
must be made with great caution since different sealants may
10.1520/F2392-04R10.
respond differently to varying conditions.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
4.4 As the true sealant strength is strongly dependent on the
Standards volume information, refer to the standard’s Document Summary page on
strength of the sealant/substrate interface, the selection of a
the ASTM website.
proper test substrate is critical. Care must be taken when
Available from American Association for Tissue Banks (AATB), 1320 Old
Chain Bridge Rd., Suite 450, McLean, VA 22101. extrapolating in vitro test results to in vivo expectations. In
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F2392 − 04 (2010)
vitro sealant optimization may not translate to expected in vivo that relatively large samples of tissue can be harvested from a
performance due to differences in substrate surface, strength, single source. Ideally, the tissue should be used within 24 h of
and elasticity. harvest and should be kept between 5 and 10°C prior to testing
if it cannot be used immediately after harvesting. Storage and
5. Apparatus
handling of tissue samples should be carried out according to
the guidelines set forth in Standards for Tissue Banking by the
5.1 Testing Machine—Atestingmachinefordeterminingthe
American Association of Tissue Banks. The specimens should
sealant strength and system failure mechanism and comprising
be brought to the test temperature or other prescribed tempera-
essentially the following:
ture (such as body temperature) prior to application of the
5.1.1 Test Fixture—A stationary fixture containing the test
sealant.
substrate and applied sealant. Fluid flows into the fixture at a
6.3.2 Fixed tissue should not be used since it has been
fixed rate, allowing for the pressurization of the sealed sub-
demonstrated that fixatives cause large alterations in the
strate.
mechanical properties of the tissue and it is probable that the
5.1.2 Positive Displacement Fluid Pump—A pump provid-
adhesive strength would be affected as well.
ing a constant flow of fluid to the test fixture. The pump must
6.3.3 Ifthetargetorganisofasizeorgeometry,orboth,that
be capable of constant flow at pressures of interest. Syringe
does not allow fabrication of test samples, a tissue of similar
pumps are particularly well suited for this type of testing since
origin but larger size should be used.
they do not cause pulsatile flow. Peristaltic pumps have also
6.3.4 The thickness of the tissue sample should be mini-
been used successfully since the pump tubing tends to dampen
mized and should not exceed 5 mm. Thicker samples will lead
pulsations.
to distortion of the substrate and may leak in the test fixture.
NOTE 1—Saline is the typical fluid of choice. When air is used, a
Also, thicker samples will lead to sealant adherence on the
reduction in pressurization rate is expected due to gas compressibility.
insides of the hole itself, possibly leading to different failure
5.1.3 Pressure gage—Consisting of a gage and method of
mechanisms. It is also important that the thickness be as
capturing peak pressures. System sampling rate should be
uniform as possible.
adequate to capture peak burst pressures. Sensitivity and
6.4 Substrates for Quality Control Testing:
precision should result in less than 1 % error. The burst test
6.4.1 For testing that is undertaken as part of a quality
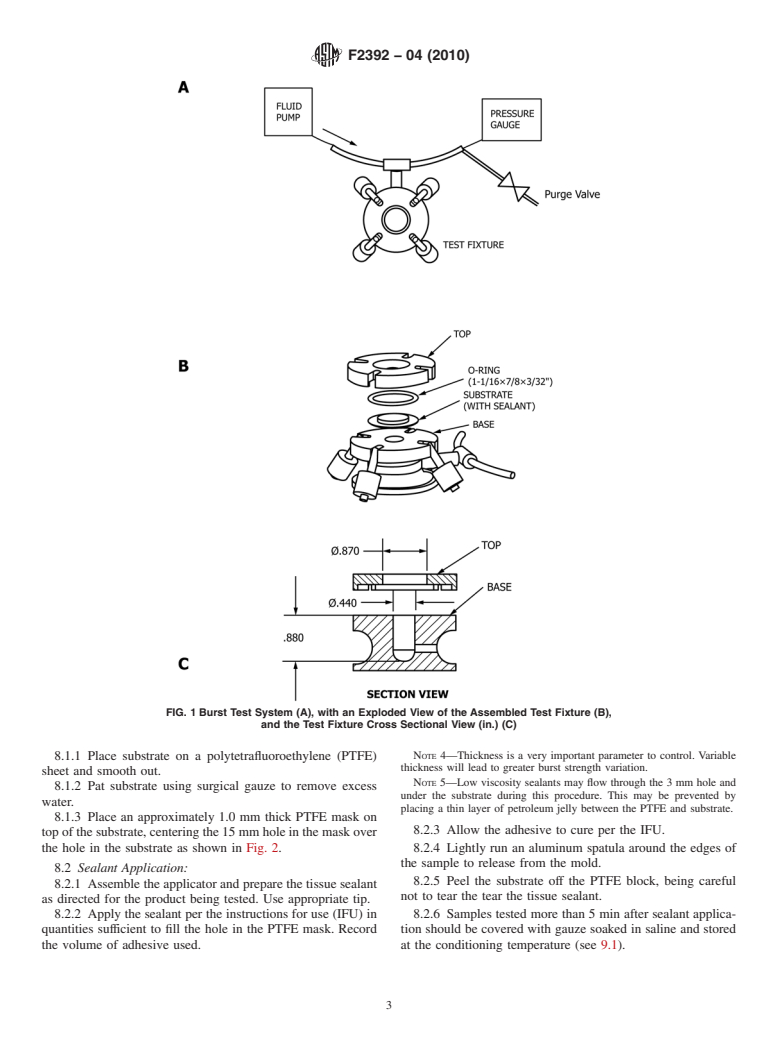
system is shown in Fig. 1. The system (A) consists of a fluid
control process in the manufacturing of a tissue sealant, the use
pump, a test fixture, and pressure gage connected by rigid
of freshly harvested tissue is highly inconvenient and may also
plastic tubing. The test fixture (B) consists of a base, O-ring,
lead to unacceptable variation in the test results, especially if
and top.
the failure occurs in the adherend (substrate failure). Since the
5.2 Temperature-controlling Equipment—Must be capable
purpose of quality control testing is to demonstrate consistency
of maintaining the test temperature to 62°C. If ambient
in the device, substitution of a model substrate is preferred so
laboratoryconditionsareemployed,thesamedegreeofcontrol
long as it is demonstrated that the sealant does bond to the
is required.Awater bath or environmental chamber capable of
adherend.Sincethebursttestfailuremechanismcandependon
maintaining 37°C is required for testing on tissue substrates.
the amount of substra
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.