ASTM F382-99(2008)
(Test Method)Standard Specification and Test Method for Metallic Bone Plates
Standard Specification and Test Method for Metallic Bone Plates
ABSTRACT
This specification and test method establishes the consistent methods for classifying, and defining the geometric and performance characteristics of five types (cloverleaf, cobra head, reconstruction, straight, and tubular) of metallic bone plates used in the surgical internal fixation of the skeletal system. Also presented here are catalogs of standard specifications for material, labeling, and handling requirements, and standard test methods for measuring performance related mechanical (single cycle bend and bend fatigue) characteristics determined to be important to the in vivo performance of bone plates. This neither defines the levels of performance or case-specific clinical performance for bone plates, nor describes specific designs for bone plates.
SIGNIFICANCE AND USE
A2.5.1 The test method establishes a uniform four-point bending fatigue test to characterize and compare the fatigue performance of different bone plate designs. This test method may be used to determine a bone plate's fatigue life at either a specific or over a range of maximum bending moment conditions. Additionally, the test method may be alternatively used to estimate a bone plate's fatigue strength for a specified number of fatigue cycles.
A2.5.2 The test method utilizes a simplified bone plate load model that may not be exactly representative of the in-situ loading configuration. The user should note that the test results generated by this test method can not be used to directly predict the in vivo performance of the bone plate being tested. The data generated from this test method can be used to conduct relative comparisons of different bone plate designs.
A2.5.3 This test method may not be appropriate for all types of implant applications. The user is cautioned to consider the appropriateness of the method in view of the devices being tested and their potential application.
A2.5.4 This test method assumes that the bone plate is manufactured from a material that exhibits linear-elastic material behavior. Therefore, the method is not applicable for testing bone plates made from materials that exhibit non-linear elastic behavior.
A2.5.5 This test method is restricted to the testing of bone plates within the materials' linear-elastic range. Therefore, the test method is not applicable for testing bone plates under conditions that would approach or exceed the bending strength of the bone plate being tested.
SCOPE
1.1 This specification and test method is intended to provide a comprehensive reference for bone plates used in the surgical internal fixation of the skeletal system. The standard establishes consistent methods to classify, define the geometric characteristics, and performance characteristics of bone plates. The standard also presents a catalog of standard specifications that specify material; labeling and handling requirements; and standard test methods for measuring performance related mechanical characteristics determined to be important to the in vivo performance of bone plates.
1.2 It is not the intention of the standard to define levels of performance or case-specific clinical performance for bone plates, as insufficient knowledge is available to predict the consequences or their use in individual patients for specific activities of daily living. Futhermore, it is not the intention of the standard to describe or specify specific designs for bone plates used in the surgical internal fixation of the skeletal system.
1.3 This document may not be appropriate for all types of bone plates. The user is cautioned to consider the appropriateness of the standard in view of a particular bone plate and its potential application.
1.4 This document includes the following test methods used in determining the following bone plate mechanical performance characteristics.
1.4.1 Standard Test Method for Single Cycle Bend Testing of Metallic Bone Plates—Annex A1.
1.4.2 Standard Test Method for Determining th...
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F 382 – 99 (Reapproved 2008)
Standard Specification and Test Method for
Metallic Bone Plates
This standard is issued under the fixed designation F382; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 Thisspecificationandtestmethodisintendedtoprovide 2.1 ASTM Standards:
a comprehensive reference for bone plates used in the surgical F67 Specification for Unalloyed Titanium, for Surgical
internal fixation of the skeletal system. The standard estab- Implant Applications (UNS R50250, UNS R50400, UNS
lishes consistent methods to classify, define the geometric R50550, UNS R50700)
characteristics, and performance characteristics of bone plates. F75 SpecificationforCobalt-28Chromium-6Molybdenum
The standard also presents a catalog of standard specifications Alloy Castings and Casting Alloy for Surgical Implants
that specify material; labeling and handling requirements; and (UNS R30075)
standard test methods for measuring performance related F86 Practice for Surface Preparation and Marking of Me-
mechanicalcharacteristicsdeterminedtobeimportanttothe in tallic Surgical Implants
vivo performance of bone plates. F 90 Specification for Wrought Cobalt-20Chromium-
1.2 It is not the intention of the standard to define levels of 15Tungsten-10Nickel Alloy for Surgical Implant Applica-
performance or case-specific clinical performance for bone tions (UNS R30605)
plates, as insufficient knowledge is available to predict the F136 Specification for Wrought Titanium-6Aluminum-
consequences or their use in individual patients for specific 4Vanadium ELI (Extra Low Interstitial)Alloy for Surgical
activities of daily living. Futhermore, it is not the intention of Implant Applications (UNS R56401)
the standard to describe or specify specific designs for bone F138 Specification for Wrought 18Chromium-14Nickel-
plates used in the surgical internal fixation of the skeletal 2.5Molybdenum Stainless Steel Bar and Wire for Surgical
system. Implants (UNS S31673)
1.3 This document may not be appropriate for all types of F139 Specification for Wrought 18Chromium-14Nickel-
bone plates. The user is cautioned to consider the appropriate- 2.5Molybdenum Stainless Steel Sheet and Strip for Surgi-
ness of the standard in view of a particular bone plate and its cal Implants (UNS S31673)
potential application. F543 Specification and Test Methods for Metallic Medical
1.4 Thisdocumentincludesthefollowingtestmethodsused Bone Screws
in determining the following bone plate mechanical perfor- F565 Practice for Care and Handling of Orthopedic Im-
mance characteristics. plants and Instruments
1.4.1 Standard Test Method for Single Cycle Bend Testing F620 Specification for Alpha Plus Beta Titanium Alloy
of Metallic Bone Plates—Annex A1. Forgings for Surgical Implants
1.4.2 Standard Test Method for Determining the Bending F621 SpecificationforStainlessSteelForgingsforSurgical
Fatigue Properties Of Metallic Bone Plates—Annex A2. Implants
1.5 The values stated in SI units are to be regarded as F983 Practice for Permanent Marking of Orthopaedic Im-
standard. The values given in parentheses are mathematical plant Components
conversions to inch-pound units that are provided for informa- F1295 Specification for Wrought Titanium-6Aluminum-
tion only and are not considered standard. 7Niobium Alloy for Surgical Implant Applications (UNS
1.6 This standard does not purport to address all of the R56700)
safety concerns, if any, associated with its use. It is the F1314 SpecificationforWroughtNitrogenStrengthened22
responsibility of the user of this standard to establish appro- Chromium−13Nickel−5Manganese−2.5Molybdenum
priate safety and health practices and determine the applica- Stainless Steel Alloy Bar and Wire for Surgical Implants
bility of regulatory limitations prior to use. (UNS S20910)
This specification and test method is under the jurisdiction ofASTM Commit-
tee F04 on Medical and Surgical Materials and Devices and is the direct For referenced ASTM standards, visit the ASTM website, www.astm.org, or
responsibility of Subcommittee F04.21 on Osteosynthesis. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Current edition approved Nov. 1, 2008. Published November 2008. Originally Standards volume information, refer to the standard’s Document Summary page on
´1
approved in 1973. Last previous edition approved in 2003 as F 382–99(2003) . the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F 382 – 99 (2008)
F1472 Specification for Wrought Titanium-6Aluminum- shown in Fig. 1a, 1b, and Fig. 2. For a bone plate with a
4VanadiumAlloy for Surgical ImplantApplications (UNS crescentsection,thethicknessismeasuredatthethickestpoint
R56400) along the section.
F1713 Specification for Wrought Titanium-13Niobium- 3.1.5 bone plate width, w (mm)—thelineardimensionofthe
13Zirconium Alloy for Surgical Implant Applications bone plate measured perpendicular to both the length and
(UNS R58130) thickness axes as shown in Fig. 2.
2.2 ISO Standard: 3.1.6 contouring—the manipulation and bending of a bone
FDIS14602 Non-active surgical implants—Implants for plate, either pre-operatively or intra-operatively, to match the
Osteosynthesis particular requirements. anatomic geometry of the intended fixation location.
3.1.7 crescent section—a bone plate cross-section shape
3. Terminology
(perpendicular to the long axis of the bone plate) where the
3.1 Definitions—Geometric:
thickness is not constant along the section. Typically the
3.1.1 auto compression—a type of bone plate that by its section is thickest along the bone plate’s centerline and tapers
design can generate a compressive force between adjacent
to a smaller thickness at the bone plate’s edges (see Fig. 1b).
unconnected bone fragments through the use of one or more
3.1.8 uniform width—referring to a bone plate where the
ramped holes or another type of slot geometry. This ramp or width is constant along the bone plate’s length.
slot geometry contacts the underside of the screw head, and
3.2 Definitions—Mechanical/Structural:
induces compressive force as the screw is inserted and tight- 3.2.1 bending stiffness, K (N/mm)— of a bone plate, the
ened to the bone plate.
maximum slope of the linear elastic portion of the load versus
3.1.2 bone plate—a metallic device with two or more holes load-point displacement curve for a bone plate when tested
or slot(s), or both, and a cross section that consists of at least
according to the test method of Annex A1.
two dimensions (width and thickness) which generally are not 3.2.2 bending strength (N-m)— of a bone plate,thebending
the same in magnitude. The device is intended to provide
moment necessary to produce a 0.2% offset displacement in
alignmentandfixationoftwoormorebonesections,primarily the bone plate when tested as described in Annex A1.
byspanningthefractureordefect.Thedeviceistypicallyfixed
3.2.3 bending structural stiffness, El (N-m )—of a bone
to the bone through the use of bone screws or cerclage wire.A plate, the bone plate’s normalized effective bending stiffness
partial list of general types of bone plates is given in Section
that takes into consideration the effects of the test setup’s
4.1.
FIG. 1 Bone Plate Cross-sections
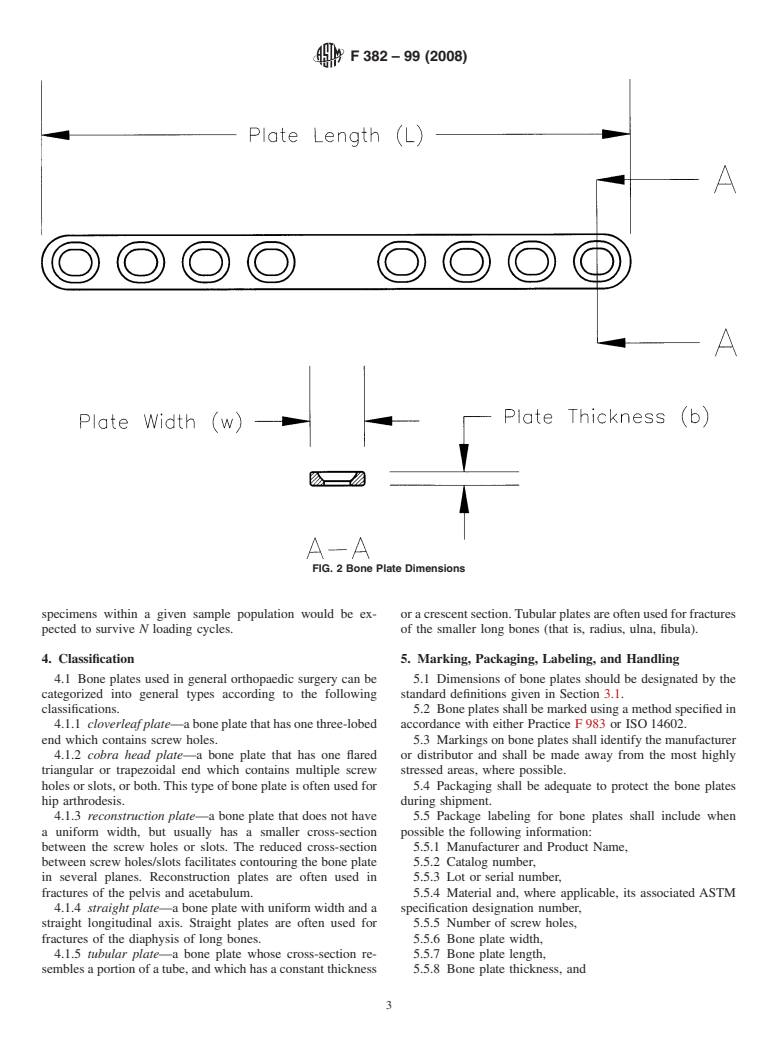
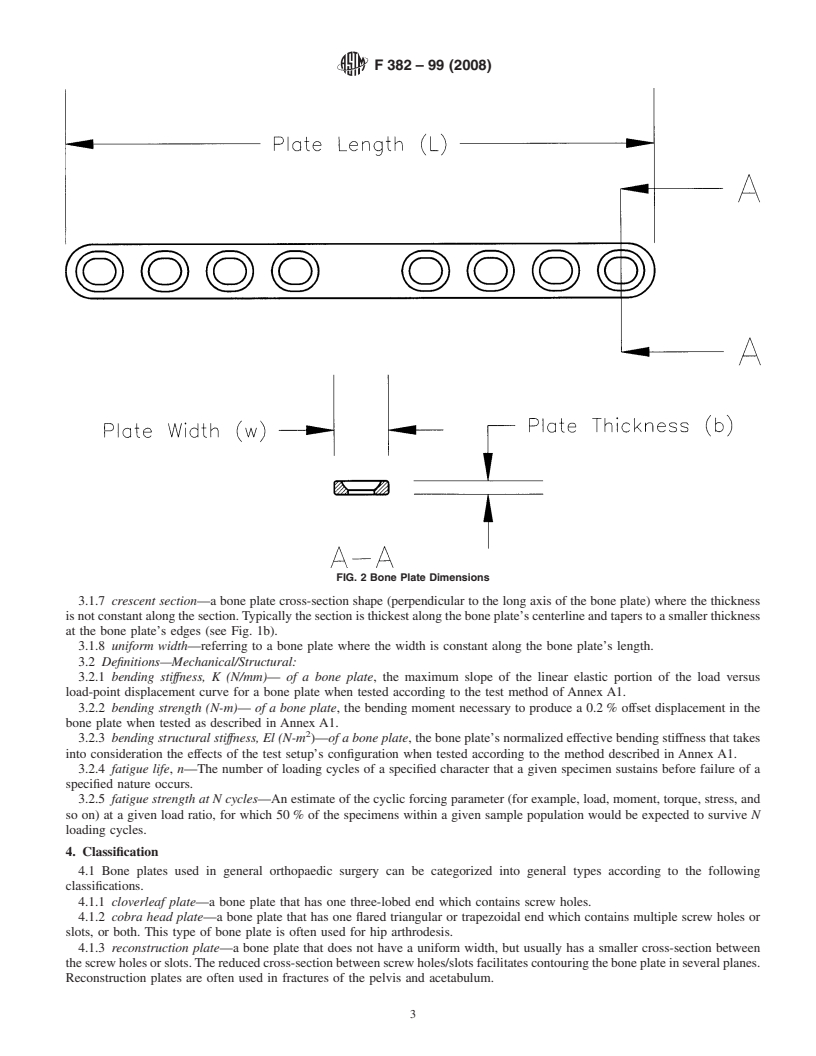
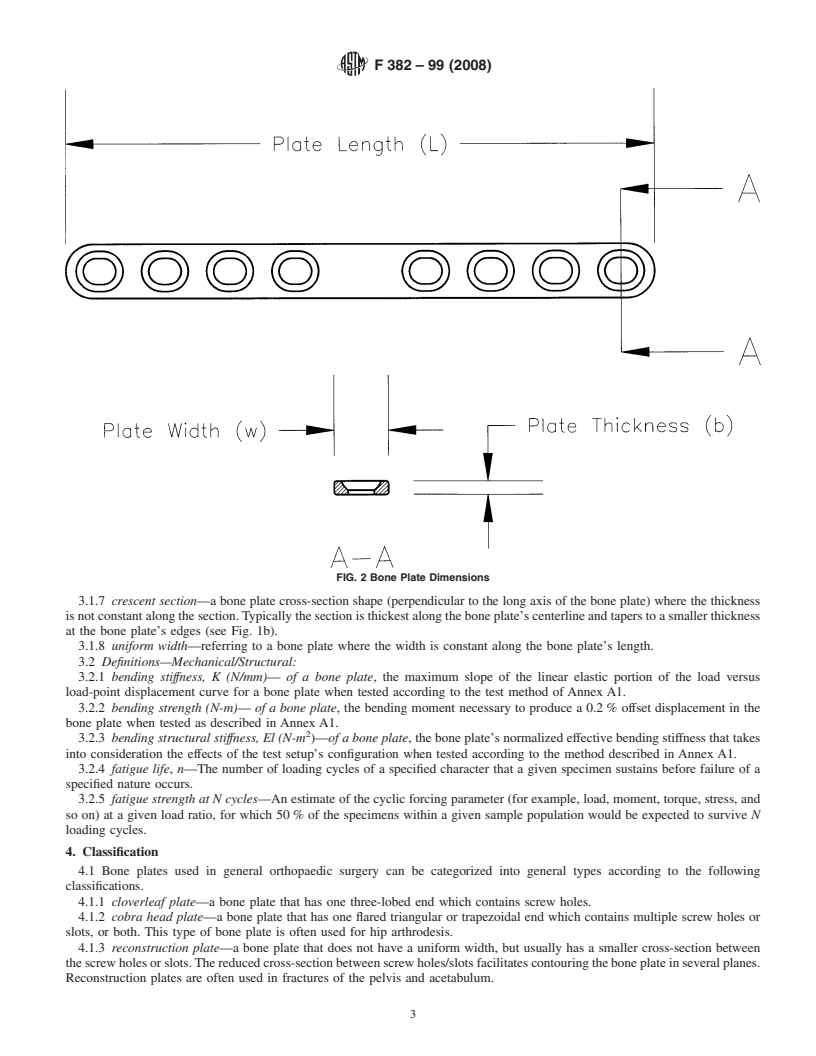
3.1.3 bone plate length, L (mm)—the linear dimension of configurationwhentestedaccordingtothemethoddescribedin
the bone plate measured along the longitudinal axis as illus- Annex A1.
trated in Fig. 2. 3.2.4 fatigue life, n—The number of loading cycles of a
3.1.4 bone plate thickness, b (mm)—thelineardimensionof
specified character that a given specimen sustains before
the bone plate measured parallel to the screw hole axis as failure of a specified nature occurs.
3.2.5 fatigue strength at N cycles—Anestimateofthecyclic
3 forcing parameter (for example, load, moment, torque, stress,
Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
4th Floor, New York, NY 10036, http://www.ansi.org. and so on) at a given load ratio, for which 50% of the
F 382 – 99 (2008)
FIG. 2 Bone Plate Dimensions
specimens within a given sample population would be ex- oracrescentsection.Tubularplatesareoftenusedforfractures
pected to survive N loading cycles. of the smaller long bones (that is, radius, ulna, fibula).
4. Classification 5. Marking, Packaging, Labeling, and Handling
4.1 Bone plates used in general orthopaedic surgery can be 5.1 Dimensions of bone plates should be designated by the
categorized into general types according to the following standard definitions given in Section 3.1.
classifications. 5.2 Boneplatesshallbemarkedusingamethodspecifiedin
4.1.1 cloverleaf plate—aboneplatethathasonethree-lobed accordance with either Practice F983 or ISO14602.
end which contains screw holes. 5.3 Markingsonboneplatesshallidentifythemanufacturer
4.1.2 cobra head plate—a bone plate that has one flared or distributor and shall be made away from the most highly
triangular or trapezoidal end which contains multiple screw stressed areas, where possible.
holesorslots,orboth.Thistypeofboneplateisoftenusedfor 5.4 Packaging shall be adequate to protect the bone plates
hip arthrodesis. during shipment.
4.1.3 reconstruction plate—a bone plate that does not have 5.5 Package labeling for bone plates shall include when
a uniform width, but usually has a smaller cross-section possible the following information:
between the screw holes or slots. The reduced cross-section 5.5.1 Manufacturer and Product Name,
between screw holes/slots facilitates contouring the bone plate 5.5.2 Catalog number,
in several planes. Reconstruction plates are often used in 5.5.3 Lot or serial number,
fractures of the pelvis and acetabulum. 5.5.4 Material and, where applicable, its associated ASTM
4.1.4 straight plate—a bone plate with uniform width and a specification designation number,
straight longitudinal axis. Straight plates are often used for 5.5.5 Number of screw holes,
fractures of the diaphysis of long bones. 5.5.6 Bone plate width,
4.1.5 tubular plate—a bone plate whose cross-section re- 5.5.7 Bone plate length,
semblesaportionofatube,andwhichhasaconstantthickness 5.5.8 Bone plate thickness, and
F 382 – 99 (2008)
5.5.9 ASTM specification designation number. 7.2 bending properties—a critical characteristic of bone
5.6 Bone plates should be cared for and handled in accor- platesfororthopedicapplicationssincetheboneplateprovides
dance with Practice F565, as appropriate.
theprimarymeansofstabilizingthebonefragments.Addition-
ally, the bending stiffness of the bone plate may directly affect
6. Materials
the rate and ability of healing.
6.1 AllboneplatesmadeofmaterialswhichhaveanASTM
7.2.1 The relevant bending properties (bending stiffness,
committee F04 standard designation shall meet those require-
bending structural stiffness, and bending strength) shall be
ments given in the ASTM standards. A majority of materials
determined using the standard test method of Annex A1.
having ASTM specifications can be found in the list of
7.2.2 Determine the relevant bending fatigue properties
referenced ASTM standards of Section 2.1.
according to the methods described in Annex A2.
6.2 BoneplatesofforgedSpecificationF136shallmeetthe
requirements of specification F620.
8. Keywords
6.3 BoneplatesofforgedSpecificationF138shallmeetthe
requirements of specification F621.
8.1 bendtesting—surgicalimplants;fatiguetest;boneplate;
orthopedicmedicaldevices—boneplates;surgicaldevices;test
7. General Requirements and Performance
methods—surgical implants
Considerations
7.1 geometric considerations—boneplatesthatareintended
to be used with bone screws shall have design features (screw
holesorslots)thatconformorappropriatelyfitthecorrespond-
ing bone screw.
ANNEXES
A1. STANDARD TEST METHOD FOR SINGLE CYCLE BEND TESTING OF METALLIC BONE PLATES
A1.1 Scope: A1.3.1.1 0.2 % offset displacement, q (mm)—permanent
deformationequalto0.2%ofthecenterloadingspandistance.
A1.1.1 This test method describes methods for single cycle
(point B in Fig. A1.1).
bend testing in order to determine intrinsic, structural proper-
A1.3.1.2 bending strength (N-m)—of a bone plate, the
ties of metallic bone plates. The test method measures the
bending moment necessary to produce a 0.2% offset displace-
bending stiffness, bending structural stiffness, and bending
mentintheboneplatewhentestedasdescribedinSectionA1.8
strength of bone plates.
(the bending moment corresponding to point D in Fig.A1.1.).
A1.1.2 This test method is intended to provide a means of
Iftheboneplatefracturesbeforetheproofpointisattainedthe
mechanicallycharacterizedifferentboneplatedesigns.Itisnot
bending strength shall be defined as the bending moment at
the intention of this standard to define levels of performance
fracture.
forboneplatesasinsufficientknowledgeisavailabletopredict
A1.3.1.3 bending structural stiffness, (EI ) (N-m )—of a
e
the consequences of the use of particular bone plate designs.
bone plate, the bone plate’s normalized effective bending
A1.1.3 Units—The values stated in SI units are to be
regarded as standard. No other units of measurement are
included in this standard.
A1.1.4 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use.
NOTE A1.1—There is currently an ISO standard (ISO 9585—Implants
for Surgery—Determination of Bending Strength and Stiffness of Bone
Plates) that is similar, but not equivalent to this test method.
A1.2 Referenced Documents:
A1.2.1 ASTM Standards :
E4 Practices for Load Verification of Testing Machines
E122 Practice for Choice of Sample Size to Estimate the
Average Quality of a Lot or Process
A1.3 Terminology:
FIG. A1.1 Diagram Illustrating Methods For Determining Bending
A1.3.1 Definitions: Properties of Bone Plates
F 382 – 99 (2008)
stiffness that takes into consideration the effects of the test
setup’s configuration. For this test method, the bending struc-
tural stiffness is determined from the single cycle bending
response of the bone plate and the testing configuration.
A1.3.1.4 bending stiffness, K (N/mm)—of a bone plate, the
maximum slope of the linear elastic
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
´1
Designation:F382–99 (Reapproved 2003) Designation: F 382 – 99 (Reapproved 2008)
Standard Specification and Test Method for
Metallic Bone Plates
This standard is issued under the fixed designation F 382; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
´ NOTE—Editorial changes were made throughout in December 2004.
1. Scope
1.1 This specification and test method is intended to provide a comprehensive reference for bone plates used in the surgical
internalfixationoftheskeletalsystem.Thestandardestablishesconsistentmethodstoclassify,definethegeometriccharacteristics,
andperformancecharacteristicsofboneplates.Thestandardalsopresentsacatalogofstandardspecificationsthatspecifymaterial;
labeling and handling requirements; and standard test methods for measuring performance related mechanical characteristics
determined to be important to the in vivo performance of bone plates.
1.2 It is not the intention of the standard to define levels of performance or case-specific clinical performance for bone plates,
as insufficient knowledge is available to predict the consequences or their use in individual patients for specific activities of daily
living. Futhermore, it is not the intention of the standard to describe or specify specific designs for bone plates used in the surgical
internal fixation of the skeletal system.
1.3 This document may not be appropriate for all types of bone plates. The user is cautioned to consider the appropriateness
of the standard in view of a particular bone plate and its potential application.
1.4 This document includes the following test methods used in determining the following bone plate mechanical performance
characteristics.
1.4.1 Standard Test Method for Single Cycle Bend Testing of Metallic Bone Plates—Annex A1.
1.4.2 Standard Test Method for Determining the Bending Fatigue Properties Of Metallic Bone Plates—Annex A2.
1.5Unless otherwise indicated, the values stated in SI units shall be regarded as the standard.
1.5 The values stated in SI units are to be regarded as standard. The values given in parentheses are mathematical conversions
to inch-pound units that are provided for information only and are not considered standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
F67 Specification for UnalloyedTitanium for Surgical ImplantApplicationsSpecification for UnalloyedTitanium, for Surgical
Implant Applications (UNS R50250, UNS R50400, UNS R50550, UNS R50700)
F75 Specification for Cobalt-28 Chromium-6 Molybdenum Alloy Castings and Casting Alloy for Surgical Implants (UNS
R30075)
F86 Practice for Surface Preparation and Marking of Metallic Surgical Implants
F90 Specification for Wrought Cobalt-20Chromium-15Tungsten-10Nickel Alloy for Surgical Implant Applications (UNS
R56401)R30605)
F 136 Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant
Applications (UNS R56401)
F 138 Specification for Wrought 18Chromium-14Nickel-2.5Molybdenum Stainless Steel Bar and Wire for Surgical Implants
(UNS S31673)
F 139 Specification for Wrought 18Chromium-14Nickel-2.5Molybdenum Stainless Steel Sheet and Strip for Surgical Implants
(UNS S31673)
This specification and test method is under the jurisdiction ofASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of
Subcommittee F04.21 on Osteosynthesis.
Current edition approved Apr. 10, 2003.Nov. 1, 2008. Published May 2003.November 2008. Originally approved in 1973. Last previous edition approved in 19992003
´1
as F 382 – 99(2003) .
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F 382 – 99 (2008)
F 543 Specification and Test Methods for Metallic Medical Bone Screws
F 565 Practice for Care and Handling of Orthopedic Implants and Instruments
F 620 Specification for Alpha Plus Beta Titanium Alloy Forgings for Surgical Implants
F 621 Specification for Stainless Steel Forgings for Surgical Implants
F 983 Practice for Permanent Marking of Orthopaedic Implant Components
F 1295 Specification for Wrought Titanium-6Aluminum-7Niobium Alloy for Surgical Implant Applications (UNS R56700)
F 1314 Wrought Nitrogen Strengthened-22Chromium-12.5Nickel-5Manganese-2.5Molybdenum Stainless Steel Bar and Wire
for Surgical Implants (UNS S20910) Specification for Wrought Nitrogen Strengthened 22 Chromium 13 Nickel 5 Manganese
2.5 Molybdenum Stainless Steel Alloy Bar and Wire for Surgical Implants (UNS S20910)
F 1472 Specification for Alpha Plus Beta Titanium Alloy Forgings for Surgical Implants Specification for Wrought
Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications (UNS R56400)
F 1713 Specification for Wrought Titanium-13Niobium-13Zirconium Alloy for Surgical Implant Applications (UNS R58130)
2.2 ISO Standard:
FDIS 14602 Non-active surgical implants—Implants for Osteosynthesis particular requirements.
3. Terminology
3.1 Definitions—Geometric:
3.1.1 auto compression—atypeofboneplatethatbyitsdesigncangenerateacompressiveforcebetweenadjacentunconnected
bone fragments through the use of one or more ramped holes or another type of slot geometry.This ramp or slot geometry contacts
the underside of the screw head, and induces compressive force as the screw is inserted and tightened to the bone plate.
3.1.2 bone plate—a metallic device with two or more holes or slot(s), or both, and a cross section that consists of at least two
dimensions (width and thickness) which generally are not the same in magnitude.The device is intended to provide alignment and
fixationoftwoormorebonesections,primarilybyspanningthefractureordefect.Thedeviceistypicallyfixedtothebonethrough
the use of bone screws or cerclage wire. A partial list of general types of bone plates is given in Section 4.1.
FIG. 1 Bone Plate Cross-sections
3.1.3 bone plate length, L (mm)—the linear dimension of the bone plate measured along the longitudinal axis as illustrated in
Fig. 2.
3.1.4 bone plate thickness, b (mm)—the linear dimension of the bone plate measured parallel to the screw hole axis as shown
in Fig. 1a, 1b, and Fig. 2. For a bone plate with a crescent section, the thickness is measured at the thickest point along the section.
3.1.5 bone plate width, w (mm)—thelineardimensionoftheboneplatemeasuredperpendiculartoboththelengthandthickness
axes as shown in Fig. 2.
3.1.6 contouring—the manipulation and bending of a bone plate, either pre-operatively or intra-operatively, to match the
anatomic geometry of the intended fixation location.
Available from International Standards Organization, Rue de Varembe, Case Postale 56, CH-1211, Geneva 20, Switzerland.
Available from American National Standards Institute (ANSI), 25 W. 43rd St., 4th Floor, New York, NY 10036, http://www.ansi.org.
F 382 – 99 (2008)
FIG. 2 Bone Plate Dimensions
3.1.7 crescent section—a bone plate cross-section shape (perpendicular to the long axis of the bone plate) where the thickness
isnotconstantalongthesection.Typicallythesectionisthickestalongtheboneplate’scenterlineandtaperstoasmallerthickness
at the bone plate’s edges (see Fig. 1b).
3.1.8 uniform width—referring to a bone plate where the width is constant along the bone plate’s length.
3.2 Definitions—Mechanical/Structural:
3.2.1 bending stiffness, K (N/mm)— of a bone plate, the maximum slope of the linear elastic portion of the load versus
load-point displacement curve for a bone plate when tested according to the test method of Annex A1.
3.2.2 bending strength (N-m)— of a bone plate, the bending moment necessary to produce a 0.2 % offset displacement in the
bone plate when tested as described in Annex A1.
3.2.3 bending structural stiffness, El (N-m )—of a bone plate, the bone plate’s normalized effective bending stiffness that takes
into consideration the effects of the test setup’s configuration when tested according to the method described in Annex A1.
3.2.4 fatigue life, n—The number of loading cycles of a specified character that a given specimen sustains before failure of a
specified nature occurs.
3.2.5 fatigue strength at N cycles—An estimate of the cyclic forcing parameter (for example, load, moment, torque, stress, and
so on) at a given load ratio, for which 50 % of the specimens within a given sample population would be expected to survive N
loading cycles.
4. Classification
4.1 Bone plates used in general orthopaedic surgery can be categorized into general types according to the following
classifications.
4.1.1 cloverleaf plate—a bone plate that has one three-lobed end which contains screw holes.
4.1.2 cobra head plate—a bone plate that has one flared triangular or trapezoidal end which contains multiple screw holes or
slots, or both. This type of bone plate is often used for hip arthrodesis.
4.1.3 reconstruction plate—a bone plate that does not have a uniform width, but usually has a smaller cross-section between
thescrewholesorslots.Thereducedcross-sectionbetweenscrewholes/slotsfacilitatescontouringtheboneplateinseveralplanes.
Reconstruction plates are often used in fractures of the pelvis and acetabulum.
F 382 – 99 (2008)
4.1.4 straight plate—aboneplatewithuniformwidthandastraightlongitudinalaxis.Straightplatesareoftenusedforfractures
of the diaphysis of long bones.
4.1.5 tubular plate—a bone plate whose cross-section resembles a portion of a tube, and which has a constant thickness or a
crescent section. Tubular plates are often used for fractures of the smaller long bones (that is, radius, ulna, fibula).
5. Marking, Packaging, Labeling, and Handling
5.1 Dimensions of bone plates should be designated by the standard definitions given in Section 3.1.
5.2 Bone plates shall be marked using a method specified in accordance with either Practice F 983 or ISO 14602.
5.3 Markingsonboneplatesshallidentifythemanufacturerordistributorandshallbemadeawayfromthemosthighlystressed
areas, where possible.
5.4 Packaging shall be adequate to protect the bone plates during shipment.
5.5 Package labeling for bone plates shall include when possible the following information:
5.5.1 Manufacturer and Product Name,
5.5.2 Catalog number,
5.5.3 Lot or serial number,
5.5.4 Material and, where applicable, its associated ASTM specification designation number,
5.5.5 Number of screw holes,
5.5.6 Bone plate width,
5.5.7 Bone plate length,
5.5.8 Bone plate thickness, and
5.5.9 ASTM specification designation number.
5.6 Bone plates should be cared for and handled in accordance with Practice F 565, as appropriate.
6. Materials
6.1 All bone plates made of materials which have anASTM committee F04 standard designation shall meet those requirements
given in the ASTM standards. A majority of materials having ASTM specifications can be found in the list of referenced ASTM
standards of Section 2.1.
6.2 Bone plates of forged Specification F 136 shall meet the requirements of specification F 620.
6.3 Bone plates of forged Specification F 138 shall meet the requirements of specification F 621.
7. General Requirements and Performance Considerations
7.1 geometric considerations—boneplatesthatareintendedtobeusedwithbonescrewsshallhavedesignfeatures(screwholes
or slots) that conform or appropriately fit the corresponding bone screw.
7.2 bending properties—a critical characteristic of bone plates for orthopedic applications since the bone plate provides the
primary means of stabilizing the bone fragments.Additionally, the bending stiffness of the bone plate may directly affect the rate
and ability of healing.
7.2.1 The relevant bending properties (bending stiffness, bending structural stiffness, and bending strength) shall be determined
using the standard test method of Annex A1.
7.2.2 Determine the relevant bending fatigue properties according to the methods described in Annex A2.
8. Keywords
8.1 bend testing—surgical implants; fatigue test; bone plate; orthopedic medical devices—bone plates; surgical devices; test
methods—surgical implants
ANNEXES
A1. STANDARD TEST METHOD FOR SINGLE CYCLE BEND TESTING OF METALLIC BONE PLATES
A1.1 Scope:
A1.1.1 This test method describes methods for single cycle bend testing in order to determine intrinsic, structural properties of
metallic bone plates. The test method measures the bending stiffness, bending structural stiffness, and bending strength of bone
plates.
A1.1.2 This test method is intended to provide a means of mechanically characterize different bone plate designs. It is not the
intention of this standard to define levels of performance for bone plates as insufficient knowledge is available to predict the
consequences of the use of particular bone plate designs.
A1.1.3 Units—The values stated in SI units are to be regarded as standard. No other units of measurement are included in this
standard.
A1.1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
F 382 – 99 (2008)
NOTE A1.1—There is currently an ISO standard (ISO 9585—Implants for Surgery—Determination of Bending Strength and Stiffness of Bone Plates)
that is similar, but not equivalent to this test method.
A1.2 Referenced Documents:
A1.2.1 ASTM Standards :
E 4 Practices for Load Verification of Testing Machines
E 122 Practice for Choice of Sample Size to Estimate the Average Quality of a Lot or Process
A1.3 Terminology:
A1.3.1 Definitions:
A1.3.1.1 0.2 % offset displacement, q (mm)—permanent deformation equal to 0.2 %
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
´1
Designation:F382–99 (Reapproved 2003) Designation: F 382 – 99 (Reapproved 2008)
Standard Specification and Test Method for
Metallic Bone Plates
This standard is issued under the fixed designation F 382; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
´ NOTE—Editorial changes were made throughout in December 2004.
1. Scope
1.1 This specification and test method is intended to provide a comprehensive reference for bone plates used in the surgical
internalfixationoftheskeletalsystem.Thestandardestablishesconsistentmethodstoclassify,definethegeometriccharacteristics,
andperformancecharacteristicsofboneplates.Thestandardalsopresentsacatalogofstandardspecificationsthatspecifymaterial;
labeling and handling requirements; and standard test methods for measuring performance related mechanical characteristics
determined to be important to the in vivo performance of bone plates.
1.2 It is not the intention of the standard to define levels of performance or case-specific clinical performance for bone plates,
as insufficient knowledge is available to predict the consequences or their use in individual patients for specific activities of daily
living. Futhermore, it is not the intention of the standard to describe or specify specific designs for bone plates used in the surgical
internal fixation of the skeletal system.
1.3 This document may not be appropriate for all types of bone plates. The user is cautioned to consider the appropriateness
of the standard in view of a particular bone plate and its potential application.
1.4 This document includes the following test methods used in determining the following bone plate mechanical performance
characteristics.
1.4.1 Standard Test Method for Single Cycle Bend Testing of Metallic Bone Plates—Annex A1.
1.4.2 Standard Test Method for Determining the Bending Fatigue Properties Of Metallic Bone Plates—Annex A2.
1.5Unless otherwise indicated, the values stated in SI units shall be regarded as the standard.
1.5 The values stated in SI units are to be regarded as standard. The values given in parentheses are mathematical conversions
to inch-pound units that are provided for information only and are not considered standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
F67Specification for Unalloyed Titanium for Surgical Implant Applications 67 Specification for Unalloyed Titanium, for
Surgical Implant Applications (UNS R50250, UNS R50400, UNS R50550, UNS R50700)
F 75 Specification for Cobalt-28 Chromium-6 Molybdenum Alloy Castings and Casting Alloy for Surgical Implants (UNS
R30075)
F 86 Practice for Surface Preparation and Marking of Metallic Surgical Implants
F 90 Specification for Wrought Cobalt-20Chromium-15Tungsten-10Nickel Alloy for Surgical Implant Applications (UNS
R56401) R30605)
F 136 Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant
Applications (UNS R56401)
F 138 Specification for Wrought 18Chromium-14Nickel-2.5Molybdenum Stainless Steel Bar and Wire for Surgical Implants
(UNS S31673)
F 139 Specification for Wrought 18Chromium-14Nickel-2.5Molybdenum Stainless Steel Sheet and Strip for Surgical Implants
(UNS S31673)
This specification and test method is under the jurisdiction ofASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of
Subcommittee F04.21 on Osteosynthesis.
Current edition approved Apr. 10, 2003.Nov. 1, 2008. Published May 2003.November 2008. Originally approved in 1973. Last previous edition approved in 19992003
´1
as F 382 – 99(2003) .
For referencedASTM standards, visit theASTM website, www.astm.org, or contactASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F 382 – 99 (2008)
F 543 Specification and Test Methods for Metallic Medical Bone Screws
F 565 Practice for Care and Handling of Orthopedic Implants and Instruments
F 620 Specification for Alpha Plus Beta Titanium Alloy Forgings for Surgical Implants
F 621 Specification for Stainless Steel Forgings for Surgical Implants
F 983 Practice for Permanent Marking of Orthopaedic Implant Components
F 1295 Specification for Wrought Titanium-6Aluminum-7Niobium Alloy for Surgical Implant Applications (UNS R56700)
F1314Wrought Nitrogen Strengthened-22Chromium-12.5Nickel-5Manganese-2.5Molybdenum Stainless Steel Bar andWire for
Surgical Implants (UNS S20910) 1314 Specification for Wrought Nitrogen Strengthened 22 Chromium 13 Nickel 5
Manganese 2.5 Molybdenum Stainless Steel Alloy Bar and Wire for Surgical Implants (UNS S20910)
F1472Specification for Alpha Plus Beta Titanium Alloy Forgings for Surgical Implants 1472 Specification for Wrought
Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications (UNS R56400)
F 1713 Specification for Wrought Titanium-13Niobium-13Zirconium Alloy for Surgical Implant Applications (UNS R58130)
2.2 ISO Standard:
FDIS 14602 Non-active surgical implants—Implants for Osteosynthesis particular requirements.
3. Terminology
3.1 Definitions—Geometric:
3.1.1 auto compression—atypeofboneplatethatbyitsdesigncangenerateacompressiveforcebetweenadjacentunconnected
bone fragments through the use of one or more ramped holes or another type of slot geometry.This ramp or slot geometry contacts
the underside of the screw head, and induces compressive force as the screw is inserted and tightened to the bone plate.
3.1.2 bone plate—a metallic device with two or more holes or slot(s), or both, and a cross section that consists of at least two
dimensions (width and thickness) which generally are not the same in magnitude.The device is intended to provide alignment and
fixationoftwoormorebonesections,primarilybyspanningthefractureordefect.Thedeviceistypicallyfixedtothebonethrough
the use of bone screws or cerclage wire. A partial list of general types of bone plates is given in Section 4.1.
FIG. 1 Bone Plate Cross-sections
3.1.3 bone plate length, L (mm)—the linear dimension of the bone plate measured along the longitudinal axis as illustrated in
Fig. 2.
3.1.4 bone plate thickness, b (mm)—the linear dimension of the bone plate measured parallel to the screw hole axis as shown
in Fig. 1a, 1b, and Fig. 2. For a bone plate with a crescent section, the thickness is measured at the thickest point along the section.
3.1.5 bone plate width, w (mm)—thelineardimensionoftheboneplatemeasuredperpendiculartoboththelengthandthickness
axes as shown in Fig. 2.
3.1.6 contouring—the manipulation and bending of a bone plate, either pre-operatively or intra-operatively, to match the
anatomic geometry of the intended fixation location.
Available from International Standards Organization, Rue de Varembe, Case Postale 56, CH-1211, Geneva 20, Switzerland.
Available from American National Standards Institute (ANSI), 25 W. 43rd St., 4th Floor, New York, NY 10036, http://www.ansi.org.
F 382 – 99 (2008)
FIG. 2 Bone Plate Dimensions
3.1.7 crescent section—a bone plate cross-section shape (perpendicular to the long axis of the bone plate) where the thickness
isnotconstantalongthesection.Typicallythesectionisthickestalongtheboneplate’scenterlineandtaperstoasmallerthickness
at the bone plate’s edges (see Fig. 1b).
3.1.8 uniform width—referring to a bone plate where the width is constant along the bone plate’s length.
3.2 Definitions—Mechanical/Structural:
3.2.1 bending stiffness, K (N/mm)— of a bone plate, the maximum slope of the linear elastic portion of the load versus
load-point displacement curve for a bone plate when tested according to the test method of Annex A1.
3.2.2 bending strength (N-m)— of a bone plate, the bending moment necessary to produce a 0.2 % offset displacement in the
bone plate when tested as described in Annex A1.
3.2.3 bending structural stiffness, El (N-m )—of a bone plate, the bone plate’s normalized effective bending stiffness that takes
into consideration the effects of the test setup’s configuration when tested according to the method described in Annex A1.
3.2.4 fatigue life, n—The number of loading cycles of a specified character that a given specimen sustains before failure of a
specified nature occurs.
3.2.5 fatigue strength at N cycles—An estimate of the cyclic forcing parameter (for example, load, moment, torque, stress, and
so on) at a given load ratio, for which 50 % of the specimens within a given sample population would be expected to survive N
loading cycles.
4. Classification
4.1 Bone plates used in general orthopaedic surgery can be categorized into general types according to the following
classifications.
4.1.1 cloverleaf plate—a bone plate that has one three-lobed end which contains screw holes.
4.1.2 cobra head plate—a bone plate that has one flared triangular or trapezoidal end which contains multiple screw holes or
slots, or both. This type of bone plate is often used for hip arthrodesis.
4.1.3 reconstruction plate—a bone plate that does not have a uniform width, but usually has a smaller cross-section between
thescrewholesorslots.Thereducedcross-sectionbetweenscrewholes/slotsfacilitatescontouringtheboneplateinseveralplanes.
Reconstruction plates are often used in fractures of the pelvis and acetabulum.
F 382 – 99 (2008)
4.1.4 straight plate—aboneplatewithuniformwidthandastraightlongitudinalaxis.Straightplatesareoftenusedforfractures
of the diaphysis of long bones.
4.1.5 tubular plate—a bone plate whose cross-section resembles a portion of a tube, and which has a constant thickness or a
crescent section. Tubular plates are often used for fractures of the smaller long bones (that is, radius, ulna, fibula).
5. Marking, Packaging, Labeling, and Handling
5.1 Dimensions of bone plates should be designated by the standard definitions given in Section 3.1.
5.2 Bone plates shall be marked using a method specified in accordance with either Practice F 983 or ISO 14602.
5.3 Markingsonboneplatesshallidentifythemanufacturerordistributorandshallbemadeawayfromthemosthighlystressed
areas, where possible.
5.4 Packaging shall be adequate to protect the bone plates during shipment.
5.5 Package labeling for bone plates shall include when possible the following information:
5.5.1 Manufacturer and Product Name,
5.5.2 Catalog number,
5.5.3 Lot or serial number,
5.5.4 Material and, where applicable, its associated ASTM specification designation number,
5.5.5 Number of screw holes,
5.5.6 Bone plate width,
5.5.7 Bone plate length,
5.5.8 Bone plate thickness, and
5.5.9 ASTM specification designation number.
5.6 Bone plates should be cared for and handled in accordance with Practice F 565, as appropriate.
6. Materials
6.1 All bone plates made of materials which have anASTM committee F04 standard designation shall meet those requirements
given in the ASTM standards. A majority of materials having ASTM specifications can be found in the list of referenced ASTM
standards of Section 2.1.
6.2 Bone plates of forged Specification F 136 shall meet the requirements of specification F 620.
6.3 Bone plates of forged Specification F 138 shall meet the requirements of specification F 621.
7. General Requirements and Performance Considerations
7.1 geometric considerations—boneplatesthatareintendedtobeusedwithbonescrewsshallhavedesignfeatures(screwholes
or slots) that conform or appropriately fit the corresponding bone screw.
7.2 bending properties—a critical characteristic of bone plates for orthopedic applications since the bone plate provides the
primary means of stabilizing the bone fragments.Additionally, the bending stiffness of the bone plate may directly affect the rate
and ability of healing.
7.2.1 The relevant bending properties (bending stiffness, bending structural stiffness, and bending strength) shall be determined
using the standard test method of Annex A1.
7.2.2 Determine the relevant bending fatigue properties according to the methods described in Annex A2.
8. Keywords
8.1 bend testing—surgical implants; fatigue test; bone plate; orthopedic medical devices—bone plates; surgical devices; test
methods—surgical implants
ANNEXES
A1. STANDARD TEST METHOD FOR SINGLE CYCLE BEND TESTING OF METALLIC BONE PLATES
A1.1 Scope:
A1.1.1 This test method describes methods for single cycle bend testing in order to determine intrinsic, structural properties of
metallic bone plates. The test method measures the bending stiffness, bending structural stiffness, and bending strength of bone
plates.
A1.1.2 This test method is intended to provide a means of mechanically characterize different bone plate designs. It is not the
intention of this standard to define levels of performance for bone plates as insufficient knowledge is available to predict the
consequences of the use of particular bone plate designs.
A1.1.3 Units—The values stated in SI units are to be regarded as standard. No other units of measurement are included in this
standard.
A1.1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
F 382 – 99 (2008)
NOTE A1.1—There is currently an ISO standard (ISO 9585—Implants for Surgery—Determination of Bending Strength and Stiffness of Bone Plates)
that is similar, but not equivalent to this test method.
A1.2 Referenced Documents:
A1.2.1 ASTM Standards :
E 4 Practices for Load Verification of Testing Machines
E 122 Practice for Choice of Sample Size to Estimate the Average Quality of a Lot or Process
A1.3 Terminology:
A1.3.1 Definitions:
A1.3.1.1 0.2 % offset displacement, q (mm)—permanent deformation
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.