ASTM F55-82
(Specification)Specification for Stainless Steel Bar and Wire for Surgical Implants (Withdrawn 1989)
Specification for Stainless Steel Bar and Wire for Surgical Implants (Withdrawn 1989)
General Information
Standards Content (Sample)
ASTM F55 82 O759530 0052373 2 W
~~~ ~~__ ~~ ___ ~________~ ~
______
--&QQ-d/
Designation: F 55 - 82
AMERICAN SOCIETY FOR TESTING AND MATERIAIS
1916 Race St., Phiiadelphia,Pa. 19103
Reprinted from the Annual Book of ASTM Stanaards, Copyright ASTM
if not listed in the current combined index, will appear in the next edition.
Standard Specification for
STAINLESS STEEL BAR AND WIRE FOR SURGICAL
IMPLANTS’
Thisstandard is issued under the fixed desinnation F 55: the number immediatelyfoiiowing the designationindicates the year
of onginaiadoption or, in the case of ievihn, the year of Iast revision. A number h parentheses indicates the year of 1st
reapproval. A superscript epsilon (E) indicates an editorial change since the last revKsion or reapproval.
This spec@ca;ion has teen approved for use by agemies of the Depariment of Dqénse and for Iisiing in the DoD Index of
Spec$cotions and Standards.
1. scope
editions of SpecXicationsA 484 and A 555 shali
apply-
1.1 This specification covers the require-
3.2 In the case where a confiict exists be-
ments for two compositions of stainless steeI
tween this standard and those listed in 2.1 and
bar and wire, except suture wire, used for the
2.2, this standard shall take precedence.
manufacture of surgical implants.
NOTE L-Expasure to temperatures above 60°F
4. Ordering information
(425’C) during fabrication may impair corrosion re-
4.€ Inquiries and orders for material under
sistance unless such exposure is fozowed by a solu-
tion-annealing treatment.
this specification shall include thv foliowing
information:
1.2 The values stated in inch-pound units
4.1. L Quantity (weight or number of pieces),
are to be regarded as the standard.
4.1.2 Grade (1 or 2),
2, Applicable Documents 4.1.3 ASTM designation,
4.1.4 Form (bar, wire, fme wire),
2.1 ASTMStandardx
4.1.5 Condition (see 5. i),
A262 Recommended Practices for Detecting
4.1.6 Mechanical properties (if applicable,
Susceptibility to Intergranular Attack in
for special conditions),
Stainless Steel’
4.1.7 Finish (see 5.2),
A 484 Specification for General Require-
4.1.8 Applicable dimensions including size,
ments for Stainless and Heat-Resisting
thickness, width, and length (exact, random or
Wrought Steel Products (Except Wire)’
multiples) or print number, and
A555 Specification for General Require-
4.1.9 Special requirements.
ments for Stainless and Heat-Resisting
Steel Wire2
5. Manufacture
A 75 1 Methods, Practices, and Definitions
5.1 Condition:
for Chemical Analysis of Steel Products2
E 112 Estimating the Average Grain Size of
Metals4
‘This specification is under the jurisdiction of ASTM
Committee F-4 on Medical and Surgical Materiais and De-
2.2 American Society for Quality Control
vices and is the direct reswnsibilitvof SubcommitteeF04.02
(ASQC) Standard:
on Resources.
Cl-1968 Specification of General Require-
CuEent editionapproved March 26,1982. Published June
1982. Originally p~blished as F 55 - 65 T. Lact previous edi-
ments for a Quality Program5
tion F 55 - 16.
‘Annual Book of ASTM Sfandarh, Pari 3.
3. Cenerai Requirements for Delivery
:Annual Book of ASTM Siandar&, Part 5.
Annaol Book of ASTM Sianduth, Part 11.
3.1 In addition to the requirements of this
Available from American Society for Quaiity Control,
specification, all requirements of the current
161 W. WiiscoaskAve., Milwaukee, Wis. 53203.
---------------------- Page: 1 ----------------------
ASTM F55 82 W 0757510 0052374 4
F 55
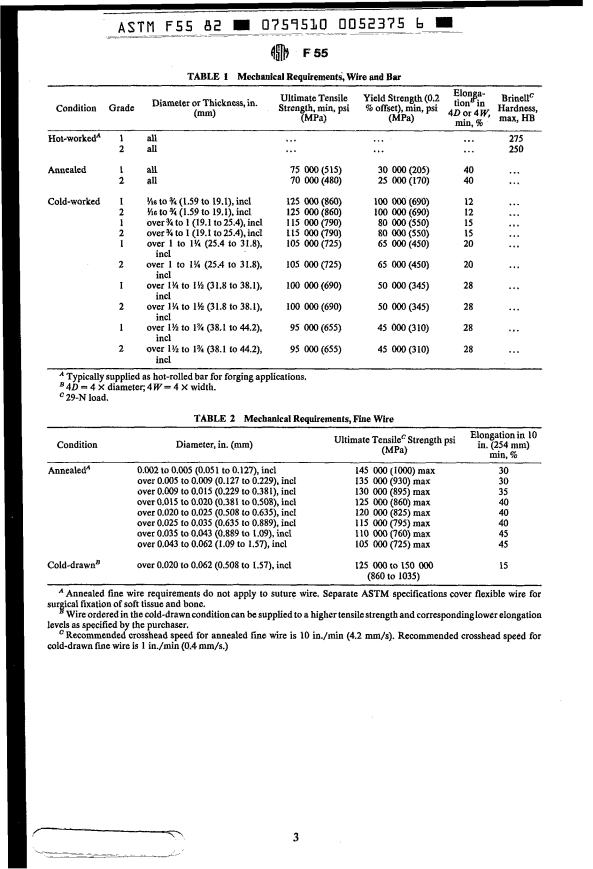
5.1.1 Bar and wire shall be furnished to the (HRC), limits may be specified. Hardness de-
termination on cold-worked material shall be
implant manufacturer; as specified, in the hot-
made on a product cross section, midway be-
worked, annealed, or cold-worked condition
tween the center and surface, if cross-section
(see Table i).
size is adequate.
5.1.2 Fine wire shall be furnished to the
implant manufacturer, as specified, in the an-
nealed, or cold-drawn condition (see Table 2). 9. SpecidTests
5.2 Finish:
9.1 The steel shall be capable of passing the
5.2.1 Types of f~sh available in bar and
intergranular corrosion susceptibility test in ac-
wire products are cold-drawn, pickled, ground,
cordance with Recommended Practices A 262,
ground and polished, or as specified in the
Practice E.
implant manufacturer‘s purchase order.
9.1.1 Samples in the hot-worked condition
5.2.2 Types of fdsh available for fine wire
shall be annealed prior to Recommended Prac-
products are cold-drawn, bright-annealed,
tices A 262, Practice E, sensitization heat treat-
pickled, ground, ground and polished, or as
ment.
specified in the impIant manufacturer’s pur-
9.2 The grain size shall be five or fmer when
chase order.
tested in accordance with Methods E 112.
9.2.1 It is preferred that samples for grain
6. Chemical Requirements
sue determination be selected after the hot-
6.1 The heat analysis shall conform to the
working operation or aft
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.