ASTM D1266-98(2003)e1
(Test Method)Standard Test Method for Sulfur in Petroleum Products (Lamp Method)
Standard Test Method for Sulfur in Petroleum Products (Lamp Method)
SIGNIFICANCE AND USE
This test method provides a means of monitoring the sulfur level of various petroleum products and additives. This knowledge can be used to predict performance, handling, or processing properties. In some cases the presence of sulfur components is beneficial to the product and monitoring the depletion of sulfur compounds provides useful information. In other cases the presence of sulfur compounds is detrimental to the processing or use of the product.
SCOPE
1.1 This test method covers the determination of total sulfur in liquid petroleum products in concentrations from 0.01 to 0.4 mass % (Note 1). A special sulfate analysis procedure is described in Annex A1 that permits the determination of sulfur in concentrations as low as 5 mg/kg.
Note 1—The comparable lamp method for the determination of sulfur in liquefied petroleum gas is described in Test Method D 2784. For the determination of sulfur in heavier petroleum products that cannot be burned in a lamp, see the bomb method (Test Method D 129) the quartz tube method (IP 63), or the high-temperature method (Test Method D 1552).
1.2 The direct burning procedure (Section 9) is applicable to the analysis of such materials as gasoline, kerosine, naphtha, and other liquids that can be burned completely in a wick lamp. The blending procedure (Section 10) is applicable to the analysis of gas oils and distillate fuel oils, naphthenic acids, alkyl phenols, high sulfur content petroleum products, and many other materials that cannot be burned satisfactorily by the direct burning procedure.
1.3 Phosphorus compounds normally present in commercial gasoline do not interfere. A correction is given for the small amount of acid resulting from the combustion of the lead anti-knock fluids in gasolines. Appreciable concentrations of acid-forming or base-forming elements from other sources interfere when the titration procedure is employed since no correction is provided in these cases.
1.4 The values stated in SI units are to be regarded as the standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
e1
Designation: D 1266 – 98 (Reapproved 2003)
Designation: 107/86
Standard Test Method for
Sulfur in Petroleum Products (Lamp Method)
This standard is issued under the fixed designation D 1266; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
e NOTE—Warning notes were editorially moved into the standard text in July 2003.
1. Scope responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
1.1 This test method covers the determination of total sulfur
bility of regulatory limitations prior to use.
in liquid petroleum products in concentrations from 0.01 to 0.4
mass % (Note 1). A special sulfate analysis procedure is
2. Referenced Documents
described inAnnexA1 that permits the determination of sulfur
2.1 ASTM Standards:
in concentrations as low as 5 mg/kg.
D 129 Test Method for Sulfur in Petroleum Products (Gen-
NOTE 1—The comparable lamp method for the determination of sulfur
eral Bomb Method)
in liquefied petroleum gas is described in Test Method D 2784. For the 3
D 1193 Specification for Reagent Water
determination of sulfur in heavier petroleum products that cannot be
D 1229 Test Method for Rubber Property—Compression
burned in a lamp, see the bomb method (Test Method D 129) the quartz
Set at Low Temperatures
tube method (IP 63), or the high-temperature method (Test Method
D 1552 TestMethodforSulfurinPetroleumProducts(High
D 1552).
Temperature Method)
1.2 Thedirectburningprocedure(Section9)isapplicableto
D 2784 Test Method for Sulfur in Liquefied Petroleum
the analysis of such materials as gasoline, kerosine, naphtha,
Gases (Oxy-Hydrogen Burner or Lamp)
andotherliquidsthatcanbeburnedcompletelyinawicklamp.
E 11 Specification for Wire Cloth and Sieves for Testing
The blending procedure (Section 10) is applicable to the
Purposes
analysis of gas oils and distillate fuel oils, naphthenic acids,
2.2 Institute of Petroleum Standard:
alkyl phenols, high sulfur content petroleum products, and
IP 63 Sulfur Content—The Quartz Tube Method
manyothermaterialsthatcannotbeburnedsatisfactorilybythe
direct burning procedure.
3. Summary of Test Method
1.3 Phosphorus compounds normally present in commercial
3.1 The sample is burned in a closed system, using a
gasoline do not interfere. A correction is given for the small
suitablelamp(Fig.1)andanartificialatmospherecomposedof
amount of acid resulting from the combustion of the lead
70 % carbon dioxide and 30 % oxygen to prevent formation of
anti-knock fluids in gasolines. Appreciable concentrations of
nitrogen oxides. The oxides of sulfur are absorbed and oxi-
acid-forming or base-forming elements from other sources
dized to sulfuric acid by means of hydrogen peroxide solution
interfere when the titration procedure is employed since no
which is then flushed with air to remove dissolved carbon
correction is provided in these cases.
dioxide. Sulfur as sulfate in the absorbent is determined
1.4 The values stated in SI units are to be regarded as the
acidimetrically by titration with standard sodium hydroxide
standard.
solution, or gravimetrically by precipitation as barium sulfate
1.5 This standard does not purport to address all of the
(see Annex A2).
safety concerns, if any, associated with its use. It is the
Annual Book of ASTM Standards, Vol 05.01.
1 3
This test method is under the jurisdiction of ASTM Committee D02 on Annual Book of ASTM Standards, Vol 11.01.
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee Annual Book of ASTM Standards, Vol 09.01.
D02.03 on Elemental Analysis. Annual Book of ASTM Standards, Vol 14.02.
Current edition approved May 10, 2003. Published July 2003. Originally Available from Institute of Petroleum (IP), 61 New Cavendish St., London,
approved in 1969. Last previous edition approved in 1998 as D 1266–98. WIG 7AR, U.K.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
e1
D 1266 – 98 (2003)
FIG. 1 Illustrative Sketch of the Assembled Lamp Unit
,
7 8
3.2 Alternatively, the sample may be burned in air, the 5.2 Cotton Wicking —Clean, unused, uniform, twisted
sulfur as sulfate in the absorbent being determined by precipi- white cotton yarn of good quality. For the burner to burn
tation as barium sulfate for weighing (see Annex A2).
aromatic samples use long staple, fine-spun, commercial fine
8,9
grade.
NOTE 2—In the absence of acid-forming or base-forming elements,
other than sulfur, results by the volumetric and gravimetric finishes 5.3 Manifold System, consisting of a vacuum manifold with
described are equivalent within the limits of precision of the method.
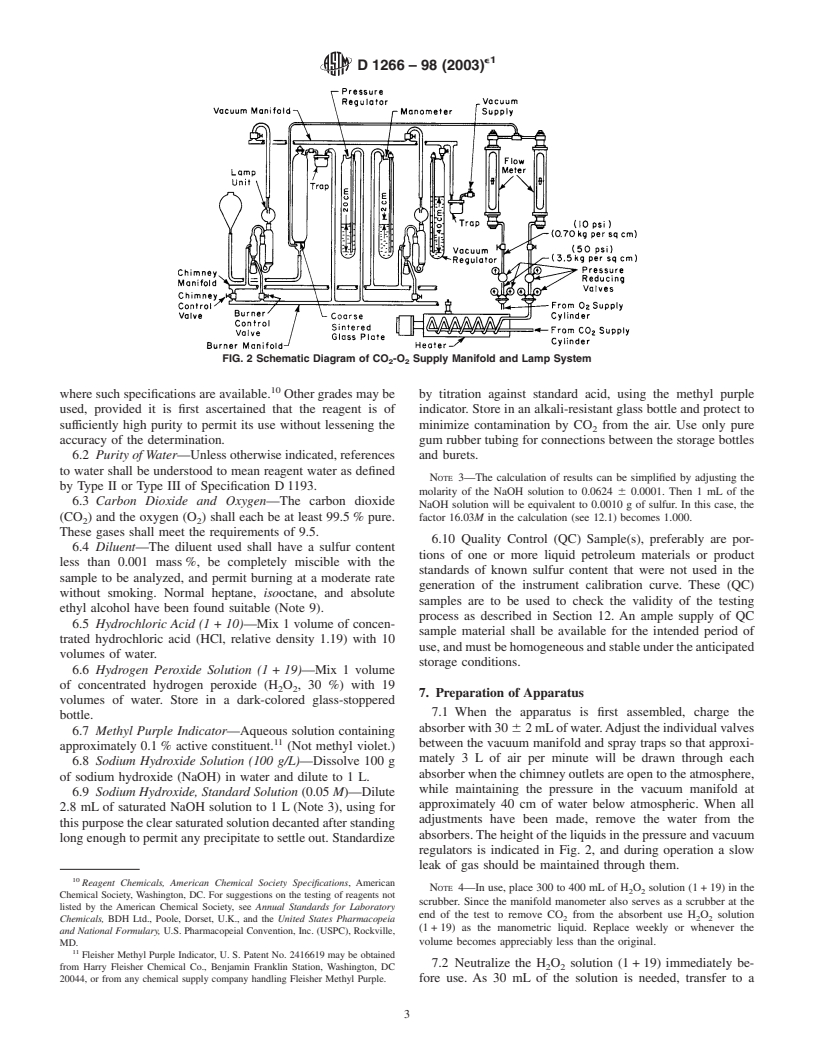
regulating device, valves, and so forth (Fig. 2) and a dual
manifold (burner and chimney) supplying a gas mixture of
3.3 For sulfur contents below 0.01 mass % it is necessary to
approximately 70 % carbon dioxide (CO ) and 30 % oxygen
determine the sulfate content in the absorber solution turbidi-
(O ) at regulated pressures. The vacuum manifold shall be
metrically as barium sulfate (see Annex A1).
connected to a pump of sufficient capacity to permit a steady
4. Significance and Use
gas flow of about 3 L/min through each absorber and to
maintain a constant manifold pressure of approximately 40 cm
4.1 This test method provides a means of monitoring the
sulfur level of various petroleum products and additives. This of water below atmospheric. The gas mixture in the chimney
knowledge can be used to predict performance, handling, or manifold shall be maintained at a nearly constant pressure of 1
processing properties. In some cases the presence of sulfur
to 2 cm of water and the burner manifold at approximately 20
components is beneficial to the product and monitoring the
cm of water. A suitable arrangement is shown in Fig. 2 and
depletion of sulfur compounds provides useful information. In
described in Annex A3, but any other similar system can be
other cases the presence of sulfur compounds is detrimental to
used. Modifications of the manifold and associated equipment
the processing or use of the product.
forburningsamplesinairareshowninFig.A2.1anddescribed
in Annex A2.
5. Apparatus
5.1 Absorbers, Chimneys, Lamps, and Spray Traps (Fig. 1),
6. Reagents and Materials
as required are described in detail in Annex A3. The standard
6.1 Purity of Reagents—Reagent grade chemicals shall be
flask and burner (Fig. A3.1) as shown is not suitable for
used in all tests. Unless otherwise indicated, it is intended that
burning highly aromatic mixtures without blending. The flask
all reagents shall conform to the specifications of the Commit-
and burner for aromatic samples (Fig. A3.1) permits burning
tee onAnalytical Reagents of theAmerican Chemical Society,
these samples directly without blending and may also be used
to burn nonaromatic samples; with this lamp, a second port
with control valve in the burner manifold is required.
The sole source of supply of cotton wicking, yarn, white, 4–strand (2 to 3
mg/cm/strand) known to the committee at this time is Koehler Instrument Co., 1595
Sycamore Ave., Bohemia, NY 11716, or the type marketed by various suppliers in
the United Kingdom as 13s/14 ends, scoured, and bleached.
If you are aware of alternative suppliers, please provide this information to
ASTM International Headquarters. Your comments will receive careful consider-
ation at a meeting of the responsible technical committee, which you may attend.
The sole source of supply of fine grade known to the committee at this time is
Thomas Scientific, P.O. Box 99, Swedesboro, NJ 08085-0099.
e1
D 1266 – 98 (2003)
FIG. 2 Schematic Diagram of CO -O Supply Manifold and Lamp System
2 2
where such specifications are available. Other grades may be by titration against standard acid, using the methyl purple
used, provided it is first ascertained that the reagent is of indicator. Store in an alkali-resistant glass bottle and protect to
sufficiently high purity to permit its use without lessening the minimize contamination by CO from the air. Use only pure
accuracy of the determination. gum rubber tubing for connections between the storage bottles
6.2 Purity of Water—Unless otherwise indicated, references and burets.
to water shall be understood to mean reagent water as defined
NOTE 3—The calculation of results can be simplified by adjusting the
by Type II or Type III of Specification D 1193.
molarity of the NaOH solution to 0.0624 6 0.0001. Then 1 mL of the
6.3 Carbon Dioxide and Oxygen—The carbon dioxide
NaOH solution will be equivalent to 0.0010 g of sulfur. In this case, the
(CO ) and the oxygen (O ) shall each be at least 99.5 % pure. factor 16.03M in the calculation (see 12.1) becomes 1.000.
2 2
These gases shall meet the requirements of 9.5.
6.10 Quality Control (QC) Sample(s), preferably are por-
6.4 Diluent—The diluent used shall have a sulfur content
tions of one or more liquid petroleum materials or product
less than 0.001 mass %, be completely miscible with the
standards of known sulfur content that were not used in the
sample to be analyzed, and permit burning at a moderate rate
generation of the instrument calibration curve. These (QC)
without smoking. Normal heptane, isooctane, and absolute
samples are to be used to check the validity of the testing
ethyl alcohol have been found suitable (Note 9).
process as described in Section 12. An ample supply of QC
6.5 Hydrochloric Acid (1 + 10)—Mix 1 volume of concen-
sample material shall be available for the intended period of
trated hydrochloric acid (HCl, relative density 1.19) with 10
use,andmustbehomogeneousandstableundertheanticipated
volumes of water.
storage conditions.
6.6 Hydrogen Peroxide Solution (1 + 19)—Mix 1 volume
of concentrated hydrogen peroxide (H O , 30 %) with 19
2 2
7. Preparation of Apparatus
volumes of water. Store in a dark-colored glass-stoppered
7.1 When the apparatus is first assembled, charge the
bottle.
absorber with 30 6 2 mLof water.Adjust the individual valves
6.7 Methyl Purple Indicator—Aqueous solution containing
between the vacuum manifold and spray traps so that approxi-
approximately 0.1 % active constituent. (Not methyl violet.)
mately 3 L of air per minute will be drawn through each
6.8 Sodium Hydroxide Solution (100 g/L)—Dissolve 100 g
absorber when the chimney outlets are open to the atmosphere,
of sodium hydroxide (NaOH) in water and dilute to 1 L.
while maintaining the pressure in the vacuum manifold at
6.9 Sodium Hydroxide, Standard Solution (0.05 M)—Dilute
approximately 40 cm of water below atmospheric. When all
2.8 mL of saturated NaOH solution to 1 L (Note 3), using for
adjustments have been made, remove the water from the
thispurposetheclearsaturatedsolutiondecantedafterstanding
absorbers.The height of the liquids in the pressure and vacuum
long enough to permit any precipitate to settle out. Standardize
regulators is indicated in Fig. 2, and during operation a slow
leak of gas should be maintained through them.
Reagent Chemicals, American Chemical Society Specifications, American
NOTE 4—In use, place 300 to 400 mL of H O solution (1 + 19) in the
2 2
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
scrubber. Since the manifold manometer also serves as a scrubber at the
listed by the American Chemical Society, see Annual Standards for Laboratory
end of the test to remove CO from the absorbent use H O solution
2 2 2
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
(1 + 19) as the manometric liquid. Replace weekly or whenever the
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
volume becomes appreciably less than the original.
MD.
Fleisher Methyl Purple Indicator, U. S. Patent No. 2416619 may be obtained
7.2 Neutralize the H O solution (1 + 19) immediately be-
from Harry Fleisher Chemical Co., Benjamin Franklin Station, Washington, DC 2 2
20044, or from any chemical supply company handling Fleisher Methyl Purple. fore use. As 30 mL of the solution is needed, transfer to a
e1
D 1266 – 98 (2003)
TABLE 1 Sample Size for Direct Combustion of Liquid Samples
beaker multiples of 30 mL sufficient for the number of
absorbers to be used simultaneously. Add 1 drop of methyl Sulfur Content, Sample Size
mass percent gmL
purpleindicatorsolutionforeach100mLofH O solutionand
2 2
Under 0.05 10 to 15 20
then add 0.05 N NaOH solution dropwise until the color
0.05 to 0.4 5to10 10
changes from purple to light green.
7.3 Introduce 30 6 2 mL of the freshly neutralized H O
2 2
solution (1 + 19) into the larger bulb of each absorber. In
for benzene and 4 mm for toluene; a slight heating of the upper
addition, for each set of samples burned, prepare an extra
end of the burner will be helpful in starting vaporization of
absorber for use as a control blank. Attach the spray traps and
heavier materials.
chimneys and connect them to their respective manifolds by
8.4 To use the standard lamp, light the wick and then slowly
means of sulfur-free rubber tubing. Close the chimney open-
admit combustion atmosphere to the burner to obtain a
ings by means of corks.
smoke-free flame. To use the burner for aromatic samples,
7.4 With the burner control valves closed, the valve to the
introduce a small amount of combustion atmosphere into the
vacuum regulator fully open, and the pressure in the vacuum
flask to provide sufficient vapor for lighting the burner. After
manifold adjusted to approximately 40 cm of water below
lighting the burner, introduce combustion atmosphere directly
atmospheric, turn on the CO and O supplies. (Warning—A
2 2
into the burner to prevent smoking and to adjust the flame size.
hazardous (explosive) condition can result if the CO supply is
If the flame is accidentally snuffed out, relight.
interrupted and the O flow is continued while samples are
8.5 A short burning period (1 to 2 min is usually sufficient)
being burned. The installation of suitable warning or control
at low flame height is necessary to allow combustion to reach
equipment is recommended.) Adjust the chimney manifold
equilibrium before the flame size can be increased without
control valve so that, at the required rate of flow through the
causing a smoky flame. In adjusting the standard lamp, the
absorbers, only a small
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.