ASTM E355-96(2007)
(Practice)Standard Practice for Gas Chromatography Terms and Relationships
Standard Practice for Gas Chromatography Terms and Relationships
ABSTRACT
This practice presents the terms, parameters, symbols, units, and relationships used in gas elution chromatography. Most of the terms described herein should also apply to other kinds of gas chromatography and various liquid column chromatographic techniques. At this time, however, they are not standardized for the latter usage. These terms include names of techniques, apparatus and reagents, parameters used in data recording and presentation of isothermal retention data, and retention parameters.
SCOPE
1.1 This practice covers primarily the terms and relationships used in gas elution chromatography. However, most of the terms should also apply to other kinds of gas chromatography and are also valid in the various liquid column chromatographic techniques, although at this time they are not standardized for the latter usage.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E355 − 96(Reapproved 2007)
Standard Practice for
Gas Chromatography Terms and Relationships
This standard is issued under the fixed designation E355; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope 2.6 Gas-Displacement Chromatography employs a desor-
bent as the carrier gas or in the carrier gas to displace a less
1.1 This practice covers primarily the terms and relation-
strongly held solute from the stationary phase which in turn
ships used in gas elution chromatography. However, most of
displaces the next less strongly held one etc., causing the
the terms should also apply to other kinds of gas chromatog-
components to emerge in the normal order, that is, least-to-
raphy and are also valid in the various liquid column chro-
most strongly absorbed.
matographic techniques, although at this time they are not
standardized for the latter usage. 2.7 Isothermal Gas Chromatography is the version of the
technique in which the column temperature is held constant
2. Names of Techniques
during the passage of the sample components through the
separation column.
2.1 Gas Chromatography, abbreviated as GC, comprises all
chromatographic methods in which the moving phase is
2.8 Programmed Temperature Gas Chromatography
gaseous. The stationary phase may be either a dry granular
(PTGC), is the version of the technique in which the column
solid or a liquid supported by the granules or by the wall of the
temperature is changed with time during the passage of the
column, or both. Separation is achieved by differences in the
sample components through the separation column. In linear
distribution of the components of a sample between the mobile
PTGC the program rate is constant during analysis. Isothermal
and stationary phases, causing them to move through the
intervals may be included in the temperature program.
column at different rates and from it at different times. In this
2.9 Programmed Flow, Pressure, or Velocity Gas Chroma-
recommended practice gas elution chromatography is implied.
tographyistheversionofthetechniqueinwhichthecarriergas
2.2 Gas-Liquid Chromatography, abbreviated as GLC, uti-
flow, pressure, or velocity is changed during analysis.
lizesaliquidasthestationaryphase,whichactsasasolventfor
2.10 Reaction Gas Chromatography is the version of the
the sample components.
technique in which the composition of the sample is changed
2.3 Gas-Solid Chromatography, abbreviated as GSC, uti-
between sample introduction and the detector.The reaction can
lizes an active solid (adsorbent) as the stationary phase.
take place upstream of the column when the chemical compo-
2.4 Gas Elution Chromatography utilizes a continuous inert sition of the individual components passing through the col-
umn differs from that of the original sample, or between the
gas flow as the carrier gas and the sample is introduced as a gas
or a liquid with a finite volume into the carrier gas stream. If column and the detector when the original sample components
are separated in the column but their chemical composition is
the sample is introduced as a liquid, it is vaporized in the
system prior to or during passage through the separation changed prior to entering the detection device.
column.
2.11 Pyrolysis Gas Chromatography is the version of reac-
2.5 Gas-Frontal Chromatography is a technique in which a tion gas chromatography in which the original sample is
decomposed by heat to more volatile components prior to
continuous stream of carrier gas mixed with sample vapor is
instantaneously replaced by a continuous stream of carrier gas passage through the separation column.
containing sample vapor at a different concentration. The
3. Apparatus
concentration profile is therefore step-shaped at the column
inlet.
3.1 Sample Inlet Systems, represent the means for introduc-
ing samples into the separation column, including the heated
zones permitting the vaporization of the introduced liquid
This practice is under the jurisdiction of ASTM Committee E13 on Molecular
Spectroscopy and Separation Science and is the direct responsibility of Subcom-
samples prior to their passage through the column. Sample
mittee E13.19 on Separation Science.
introduction can be carried out by introduction of a liquid,
Current edition approved March 1, 2007. Published March 2007. Originally
solid, or gas into the carrier-gas stream. The sample may be
approved in 1968. Last previous edition approved in 2001 as E355 – 96 (2001).
DOI: 10.1520/E0355-96R07. vaporized before or after introduction into the column.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E355 − 96 (2007)
3.1.1 Direct Inlets, rapidly vaporize the sample prior to 3.3.3 Integral Detectors, measure the accumulated quantity
enteringthecolumn.Allofthesamplevaporentersthecolumn. of sample component(s) reaching the detector.
3.3.4 Spectrometric Detectors, measure and record spectra
3.1.2 On-Column Inlets, introduce a liquid sample into the
of eluting components, such as the mass spectrum of the
column. The sample vaporizes as the column section contain-
infrared spectrum.
ing the liquid heats up after injection.
3.1.3 Split Inlets, rapidly vaporize the sample prior to
3.4 Traps, are devices for recovering sample components
entering the column. A defined fraction of the sample vapor
from the mobile phase eluting from GC columns.
entersthecolumn;theremainderleavestheinletthroughavent
at a flow rate F . The ratio of the total inlet flow (F + F )to 4. Reagents
v v c
the column flow (F ) is called the split ratio (s):
c
4.1 Carrier Gas is the Mobile Phase used to sweep or elute
F 1F the sample components through and from the column.
v c
s 5 (1)
F
c
4.2 The Stationary Phase is composed of the active immo-
bile materials within the column that selectively delay the
3.1.4 Splitless Injection, utilizes a split inlet wherein the
passage of sample components by dissolving or adsorbing
split vent flow is blocked during the injection period such that
them, or both. Inert materials that merely provide physical
most of the sample vapor enters the column. The injection
support for the stationary phase or occupy space within the
period is typically one minute. The split vent flow is reestab-
column are not part of the stationary phase.
lished afterward usually for the remainder of the run.
4.2.1 Liquid Stationary Phase is one type of stationary
3.1.5 Programmed-Temperature Vaporizers (PTV), accept a
phase which is dispersed on the solid support or the inner
liquid sample that vaporizes as the inlet system heats up after
column wall and causes the separation of the sample compo-
injection. A PTV may operate in either a split, splitless,
nents by differences in the partitioning of the sample compo-
on-column, or direct mode.
nents between the mobile and liquid phases.
3.1.6 A Retention Gap, is a section of tubing inserted
4.2.2 An Active Solid is one that has ab- or adsorptive
between the inlet and the analytical column proper. The
properties by means of which chromatographic separations
retention gap may have an inner diameter different than the
may be achieved.
analytical column. The retention gap has significantly lower
4.3 The Solid Support is the inert material that holds the
retaining power than the analytical column; in practice the
stationary (liquid) phase in intimate contact with the carrier gas
retention gap is deactivated but not coated.
flowing through it. It may consist of porous or impenetrable
3.2 Columns, consist of tubes that contain the stationary
particles or granules which hold the liquid phase and between
phase and through which the gaseous mobile phase flows.
which the carrier gas flows, or the interior wall of the column
3.2.1 Packed Columns, are filled with granular packing that
itself, or a combination of these.
is kept in place by gas-permeable plugs at both ends.
4.4 The Column Packing consists of all the material used to
3.2.2 Open-Tubular Columns, have unobstructed central
fill packed columns, including the solid support and the liquid
gasflow channels.
phase or the active solid.
3.2.2.1 Wall-Coated Open-Tubular Columns, abbreviated
4.4.1 The Liquid-Phase Loading describes the relative
WCOT columns, have the liquid phase coated directly on the
amount of liquid phase present in a packed column when the
inside, relatively smooth wall of the column tubing.
column packing consists only of the liquid phase plus the solid
3.2.2.2 Porous-Layer Open-Tubular Columns, abbreviated
support. It is usually expressed as weight percent of liquid
PLOT columns, have a solid porous layer present on the tube
phase present in the column packing:
wall but still maintain the unobstructed central gas-flow
Liquid 2 phase loading, wt% (2)
channel. This porous solid layer can either act as an adsorbent
or a support which in turn is coated with a thin film of the
amount of liquid phase 3100
~ !
liquid phase, or both.The solid layer can either be deposited on
amount of liquid phase1amount of solid support
~ !
the inside tube wall or formed by chemical means from the
4.5 Solutes are the introduced sample components that are
wall.
delayed by the column as they are eluted through it by the
3.2.2.3 Support-Coated Open-Tubular Columns, abbrevi-
carrier gas.
ated SCOT columns, refer to those PLOT Columns where the
solid layer consists of the particles of a solid support which 4.6 Unretained Substances are not delayed by the column
were deposited on the inside tube wall.
packing.
3.3 Detectors, are devices that indicate the presence of
5. Gas Chromatographic Data
eluted components in the carrier gas emerging from the
5.1 A Chromatogram is a plot of detector response against
column.
time or effluent volume. Idealized chromatograms obtained
3.3.1 Differential Concentration Detectors, measure the in-
with differential and integral detectors for an unretained
stantaneous proportion of eluted sample components in the
substance and one other component are shown in Fig. 1.
carrier gas passing through the detector.
3.3.2 Differential Mass Detectors, measure the instanta- 5.2 The definitions in this paragraph apply to chromato-
neous rate of arrival of sample components at the detector. gramsobtaineddirectlybymeansofdifferentialdetectorsorby
E355 − 96 (2007)
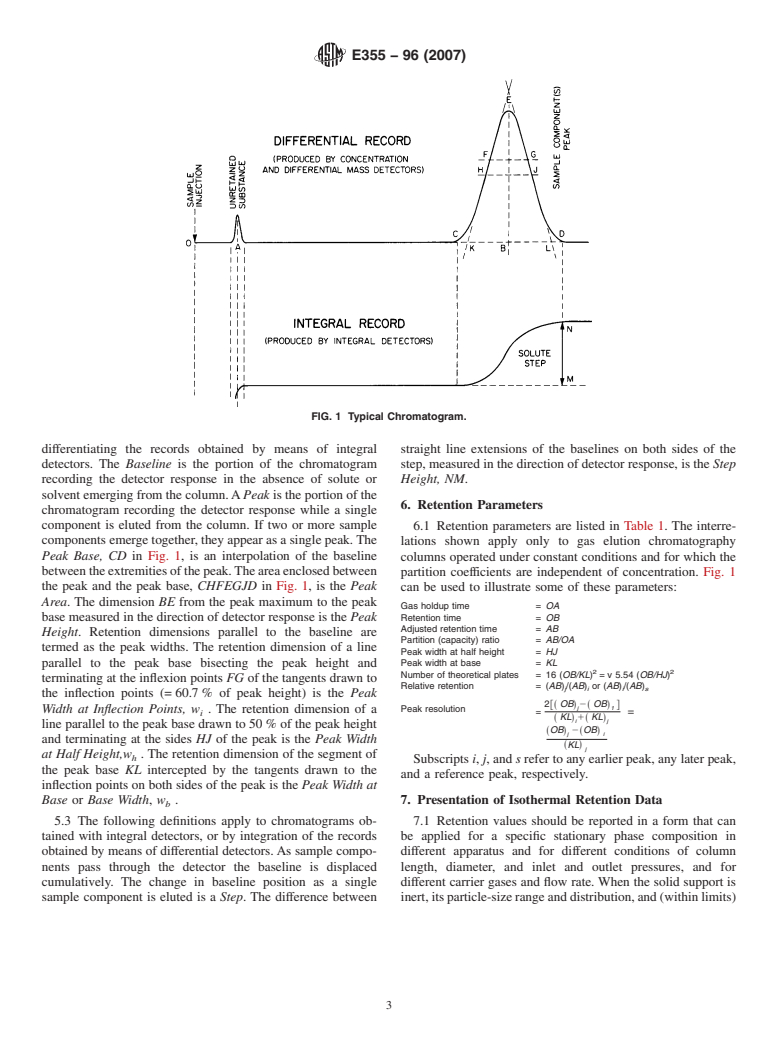
FIG. 1 Typical Chromatogram.
differentiating the records obtained by means of integral straight line extensions of the baselines on both sides of the
detectors. The Baseline is the portion of the chromatogram step, measured in the direction of detector response, is the Step
recording the detector response in the absence of solute or Height, NM.
solvent emerging from the column.APeak is the portion of the
6. Retention Parameters
chromatogram recording the detector response while a single
component is eluted from the column. If two or more sample
6.1 Retention parameters are listed in Table 1. The interre-
components emerge together, they appear as a single peak.The
lations shown apply only to gas elution chromatography
Peak Base, CD in Fig. 1, is an interpolation of the baseline columns operated under constant conditions and for which the
betweentheextremitiesofthepeak.Theareaenclosedbetween
partition coefficients are independent of concentration. Fig. 1
the peak and the peak base, CHFEGJD in Fig. 1,isthe Peak can be used to illustrate some of these parameters:
Area. The dimension BE from the peak maximum to the peak
Gas holdup time = OA
Retention time = OB
base measured in the direction of detector response is the Peak
Adjusted retention time = AB
Height. Retention dimensions parallel to the baseline are
Partition (capacity) ratio = AB/OA
termed as the peak widths. The retention dimension of a line
Peak width at half height = HJ
parallel to the peak base bisecting the peak height and Peak width at base = KL
2 2
Number of theoretical plates = 16 (OB/KL) = v 5.54 (OB/HJ)
terminating at the inflexion points FG of the tangents drawn to
Relative retention = (AB)/(AB) or (AB)/(AB)
j i i s
the inflection points (= 60.7 % of peak height) is the Peak
2fs OBd2s OBd g
j 1
Peak resolution
Width at Inflection Points, w . The retention dimension of a
= =
i
KL 1 KL
s d s d
i j
line parallel to the peak base drawn to 50 % of the peak height
sOBd 2sOBd
j i
and terminating at the sides HJ of the peak is the Peak Width
sKLd
j
at Half Height,w . The retention dimension of the segment of
h
Subscripts i, j, and s refer to any earlier peak, any later peak,
the peak base KL intercepted by the tangents drawn to the
and a reference peak, respectively.
inflection points on both sides of the peak is the Peak Width at
Base or Base Width, w . 7. Presentation of Isothermal Retention Data
b
5.3 The following definitions apply to chromatograms ob- 7.1 Retention values should be reported in a form that can
tained with integral detectors, or by integration of the records be applied for a specific stationary phase composition in
obtained by means of differential detectors.As sample compo- different apparatus and for different conditions of column
nents pass through the detector the baseline is displaced length, diameter, and inlet and outlet pressures, and for
cumulatively. The change in baseline position as a single different carrier gases and flow rate. When the solid support is
sample component is eluted is a Step. The difference between inert,itsparticle-sizerangeanddistribution,and(withinlimits)
E355 − 96 (2007)
the amount and mode of deposition of the liquid phase, may be 7.2 Retention in gas-liquid chromatography can be ex-
varied also. While the solid support is commonly assumed to pressed on an absolute basis in terms of the partition coefficient
orspecificretentionvolumeofasubstance(tacitlyassumingan
be inert, often this is not so. The physical disposition of the
liquid phase may also affect retention values (1). inert solid support). Relative retentions are more conveniently
determined, however, and they should be expressed relative to
Consequently, all components of the column packing and the
a substance which is easily available and emerges relatively
procedureforcombiningthemmustbefullyspecifiedtoenable
close to the substance of interest.
other workers to prepare identical compositions.
7.3 Retention index is another retention parameter. It is
definedrelativetotheretentionof n-alkanes,andrep
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.