ASTM F1441-92(2002)
(Specification)Standard Specification for Soft-Tissue Expander Devices
Standard Specification for Soft-Tissue Expander Devices
SCOPE
1.1 This specification covers the requirements for inflatable tissue expansion devices to be used intraoperatively or implanted for typically less than 6 months and then removed.
1.2 Limitations—This specification applies only to soft-tissue expander devices fabricated with elastomer shells. It does not necessarily cover any custom fabricated soft tissue expander device manufactured to any other specification.
1.3 The values stated in SI units are to be regarded as standard, values in parentheses are for information only.
1.4 The following statement pertains only to the test methods portion, Section 7, of this specification.This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 1441 – 92 (Reapproved 2002)
Standard Specification for
Soft-Tissue Expander Devices
This standard is issued under the fixed designation F 1441; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 3.1.1 injection port—the port through which an injection to
inflate or deflate the variable volume device is made.
1.1 This specification covers the requirements for inflatable
3.1.1.1 remote port—a port that is remote from the shell and
tissue expansion devices to be used intraoperatively or im-
attached to the shell by means of tubing.
planted for typically less than 6 months and then removed.
3.1.1.2 self-contained (integrated) port—a port that is inte-
1.2 Limitations—This specification applies only to soft-
gral to the device shell.
tissue expander devices fabricated with elastomer shells. It
3.1.2 injection surface—the area of the injection port rec-
does not necessarily cover any custom fabricated soft tissue
ommended by the manufacturer for needle insertion to inflate
expander device manufactured to any other specification.
or deflate the device.
1.3 The values stated in SI units are to be regarded as
3.1.3 needle stop—the injection port component used to
standard, values in parentheses are for information only.
limit hypodermic needle penetration through the port.
1.4 The following statement pertains only to the test meth-
3.1.4 reinforced silicone elastomer—a composite of silicone
ods portion, Section 7, of this specification. This standard does
elastomer and an embedded textile made from polyethylene
not purport to address all of the safety problems, if any,
terephthalate (Dacront) fibres.
associated with its use. It is the responsibility of the user of this
3.1.5 shell—an outer sac of the device which is comprised
standard to establish appropriate safety and health practices
of silicone elastomer (or other appropriate material).
and determine the applicability of regulatory limitations prior
3.1.6 tubing length adapter—the tissue expander compo-
to use.
nent used to connect more than one piece of remote port
2. Referenced Documents
tubing.
3.1.7 tubing/shell junction—the junction of the remote port
2.1 ASTM Standards:
tubing to the shell of the tissue expander.
D 412 Test Methods for Vulcanized Rubber and Thermo-
3.2 For other terms used in this specification see Terminol-
plastic Elastomers-Tension
ogy F 1251.
D 624 Test Method for Tear Strength of Conventional
Vulcanized Rubber and Thermoplastic Elastomers
4. Classification
D 1349 Practice for Rubber—Standard Temperatures for
2 4.1 Type I: Chronic Tissue Expansion Device—A soft tissue
Testing
expander device intended to be inflated postoperatively.
F 604 Specification for Silicon Elastomers Used in Medical
3 4.2 Type II: Immediate Tissue Expansion Device—A soft
Applications
tissue expander device only intended for intraoperative use.
F 748 Practice for Selecting Generic Biological Test Meth-
ods for Materials and Devices
5. Significance and Use
F 1251 Terminology Relating to Polymeric Biomaterials in
5.1 The devices described in this specification are intended
Medical and Surgical Devices
for use in soft tissue expansion. This specification identifies
2.2 Federal Register:
those factors felt to be important to ensure safety as it relates
Title 21, Part 820
to the device biocompatibility and the mechanical integrity of
3. Terminology the device components.
3.1 Definitions:
6. Requirements
6.1 Biocompatibility:
This specification is under the jurisdiction of ASTM Committee F04 on
6.1.1 Biological testing to ensure safety of soft tissue
Medical and Surgical Materials and Devices and is the direct responsibility of
expander devices shall be selected and conducted in accor-
Subcommittee F04.32 on Plastic and Reconstructive Surgery.
Current edition approved Dec. 15, 1992. Published February 1993.
dance with Practice F 748.
Annual Book of ASTM Standards, Vol 09.01.
6.1.2 In addition to biological testing as recommended by
Annual Book of ASTM Standards, Vol 13.01.
4 Practice F 748, other biological testing may be appropriate.
Available from U.S. Government Printing Office, Superintendent of Docu-
6.2 Physical Properties:
ments, Washington, DC 20402.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F 1441 – 92 (2002)
6.2.1 Tensile Set—Maximum set shall be less than 10 % when tested with a 25G hypodermic needle.
when tested in accordance with 7.2.1. 7.5 Overexpansion—There shall be no leakage or device
6.2.2 Breaking Force—Ultimate breaking force in tension rupture when the tissue expander is expanded (using water at
shall be no less than 11.12 N (2.5 lb) when tested in accordance ambient conditions) to 200 % of its maximum recommended
with 7.2.3. inflation volume and kept at that volume for a minimum of 10
6.2.3 Tear Resistance—Tear resistance shall be 3.5584 N min.
(0.8 lb) minimum when tested in accordance with 7.2.3. 7.6 Tubing Length Adapter Strength—Two pieces of remote
port tubing attached by means of the tubing length adapter shall
7. Test Methods
not separate when a 152.4-mm (6-in.) test specimen is stressed
7.1 Tissue expander or component designs, or both, shall
at 10 % elongation. Tubing length adapter shall not be ligated
demonstrate an acceptable response to the following tests.
in this test method.
Unless otherwise specified, the standard temperature for testing
7.7 Needle Stop Penetration—Mount a 38.1-mm (1.5-in.)
shall be 23 6 2°C (73.4 6 3.6°F). Condition the test specimens
21-gage hypodermic needle to a syringe. Insert the needle into
for at least 3 h when the test temperature is 23 6 2°C. If the
the injection port, perpendicular to the needle stop. Apply force
material is affected by moisture, maintain the relative humidity
along the axis of the needle, to push it into the needle stop. The
at 50 6 5 % and condition the specimen for at least 24 h prior
needle must fail without penetrating the needle stop.
to testing. When testing at any other temperature is required,
7.8 Fused or Adhered Joints—Requirements for adhered or
use one of the temperatures specified in Practice D 1349.
fused materials shall be critical to their integrity.
7.2 Shell—Cut the test specimens from units made by
7.8.1 Adhered or fused joints or seams that are critical to the
standard production processes including sterilization. Clean
integrity of the device envelope shall not fail when the shell
with appropriate (polar, for example, 2-propanol, or nonpolar,
adjacent to the joint is stressed to 200 % elongation for 10 s
for example, 1,1,1-trichloroethane) solvent if necessary.
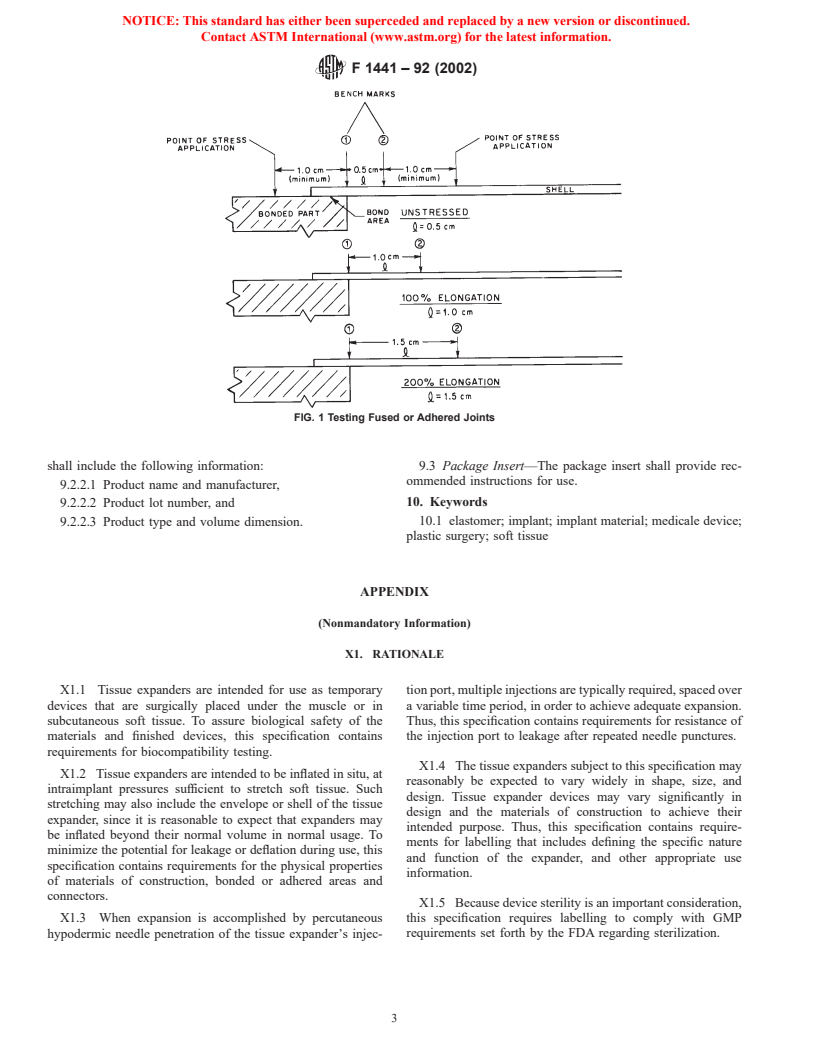
(see Fig. 1).
7.2.1 Tensile Set—At 300 % elongation, stress the test
7.8.2 Adhered or fused joints or seams that are bonded to
specimens for 3 min. Remove the load, then allow 3 min for
the device envelope, but are not critical to the envelope
relaxation. Test the
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.