ASTM F748-98
(Practice)Standard Practice for Selecting Generic Biological Test Methods for Materials and Devices
Standard Practice for Selecting Generic Biological Test Methods for Materials and Devices
SCOPE
1.1 This practice recommends generic biological test methods for materials and devices according to end-use applications. While chemical testing for extractable additives and residual monomers or residues from processing aids is necessary for most implant materials, such testing is not included as a part of this practice. The reader is cautioned that the area of materials biocompatibility testing is a rapidly evolving field, and improved methods are evolving rapidly, so this practice is by necessity only a guideline. A thorough knowledge of current techniques and research is critical to a complete evaluation of new materials.
1.2 These test protocols are intended to apply to materials and medical devices for human application. Biological evaluation of materials and devices, and related subjects such as pyrogen testing, batch testing of production lots, and so forth, are also discussed. Tests include those performed on materials, end products, and extracts. Rationale and comments on current state of the art are included for all test procedures described.
1.3 The biocompatibility of materials used in single-component or multicomponent medical devices for human use depends to a large degree on the particular nature of the end-use application. Biological reactions that are detrimental to the success of a material in one device application may have little or no bearing on the successful use of the material for a different application. It is, therefore, not possible to specify a set of biocompatibility test methods which will be necessary and sufficient to establish biocompatibility for all materials and applications.
1.4 The ethical use of research animals places the obligation on the individual investigator to determine the most efficient methods for performing the necessary testing without undue use of animals. Where adequate prior data exists to substantiate certain types of safety information, these guidelines should not be interpreted to mean that testing should be repeated unnecessarily.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F 748 – 98
Standard Practice for
Selecting Generic Biological Test Methods for Materials and
Devices
This standard is issued under the fixed designation F 748; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 1.5 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
1.1 This practice recommends generic biological test meth-
responsibility of the user of this standard to establish appro-
ods for materials and devices according to end-use applica-
priate safety and health practices and determine the applica-
tions. While chemical testing for extractable additives and
bility of regulatory limitations prior to use.
residual monomers or residues from processing aids is neces-
sary for most implant materials, such testing is not included as
2. Referenced Documents
part of this standard practice. The reader is cautioned that the
2.1 ASTM Standards:
area of materials biocompatibility testing is a rapidly evolving
E 1262 Guide for Performance of the Chinese Hamster
field, and improved methods are evolving rapidly, so this
Ovary Cell/Hypoxanthine Guanine Phosphoribosyl Trans-
standard is by necessity only a guideline. A thorough knowl-
ferase Gene Mutation Assay
edge of current techniques and research is critical to a complete
E 1280 Guide for Performance of the Mouse Lymphoma
evaluation of new materials.
Assay for Mammalian Cell Mutagenicity
1.2 These test protocols are intended to apply to materials
F 619 Standard Practice for Extraction of Medical Plastics
and medical devices for human application. Biological evalu-
F 719 Practice for Testing Biomaterials in Rabbits for
ation of materials and devices, and related subjects such as
Primary Skin Irritation
pyrogen testing, batch testing of production lots, and so on, are
F 720 Practice for Testing Guinea Pigs for Contact Aller-
also discussed. Tests include those performed on materials, end
gens: Guinea Pig Maximization Test
products, and extracts. Rationale and comments on current
F 749 Practice for Evaluating Material Extracts by Intracu-
state of the art are included for all test procedures described.
taneous Injection in the Rabbit
1.3 The biocompatibility of materials used in single or
F 750 Practic for Evaluating Material Extracts by Systemic
multicomponent medical devices for human use depends to a
Injection in the Mouse
large degree on the particular nature of the end-use application.
F 756 Practice for Assessment of the Hemolytic Properties
Biological reactions that are detrimental to the success of a
of Materials
material in one device application may have little or no bearing
F 763 Practice for Short-Term Screening of Implant Mate-
on the successful use of the material for a different application.
rials
It is, therefore, not possible to specify a set of biocompatibility
F 813 Practice for Direct Contact Cell Culture Evaluation of
test methods which will be necessary and sufficient to establish
Materials for Medical Devices
biocompatibility for all materials and applications.
F 895 Test Method for Agar Diffusion Cell Culture Screen-
1.4 The ethical use of research animals places the obligation
ing for Cytotoxicity
on the individual investigator to determine the most efficient
F 981 Practice for Assessment of Compatibility of Bioma-
methods for performing the necessary testing without undue
terials for Surgical Implants with Respect to Effect of
use of animals. Where adequate prior data exists to substantiate
Materials on Muscle and Bone
certain types of safety information, these guidelines should not
F 1027 Practice for Tissue and Cell Compatibility of Oro-
be interpreted to mean that testing should be unnecessarily
facial Prosthetic Materials and Devices
repeated.
F 1408 Practice for Subcutaneous Screening Test for Im-
plant Materials
This practice is under the jurisdiction of ASTM Committee F04 on Medical and
Surgical Devices and is direct responsibility of Subcommittee F04.16 on Biocom-
patibility Test Methods.
Current edition approved August 10, 1998. Published October 1998. Originally Annual Book of ASTM Standards, Vol 11.04.
published as F 748 – 82. Last previous edition F 748 – 95. Annual Book of ASTM Standards, Vol 13.01.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F748–98
F 1439 Guide for Performance of Lifetime Bioassay for the product may differ chemically, physically, or biologically from
Tumorigenic Potential of Implant Materials the raw materials used to fabricate the product due to process-
2.2 Other Referenced Documents: ing and this has to be considered when designing test protocols.
ISO/AAMI/ANSI 10993-1 Biological Testing of Medical For some devices it may be necessary or desirable to take
and Dental Materials and Devices - Part 1: Guidance on material test samples directly from the final device product.
Selection of Tests Samples should be fully representative of the finished product
EN 30993–1 Biological Testing of Medical and Dental in terms of processing, cleaning, packaging, sterilization, and
Materials and Devices - Part 1: Guidance on Selection of any other procedures that are performed on the materials before
Tests the device is used.
General Program Memorandum #G95-1 FDA 5.1.2 At this point preliminary material screening may be
Immunotoxicity Testing Guidance-FDA
employed, depending on the expertise of the organizations
evaluating the materials. Since preliminary screening is nor-
3. Summary of Practice
mally an option to minimize the economic impact of a
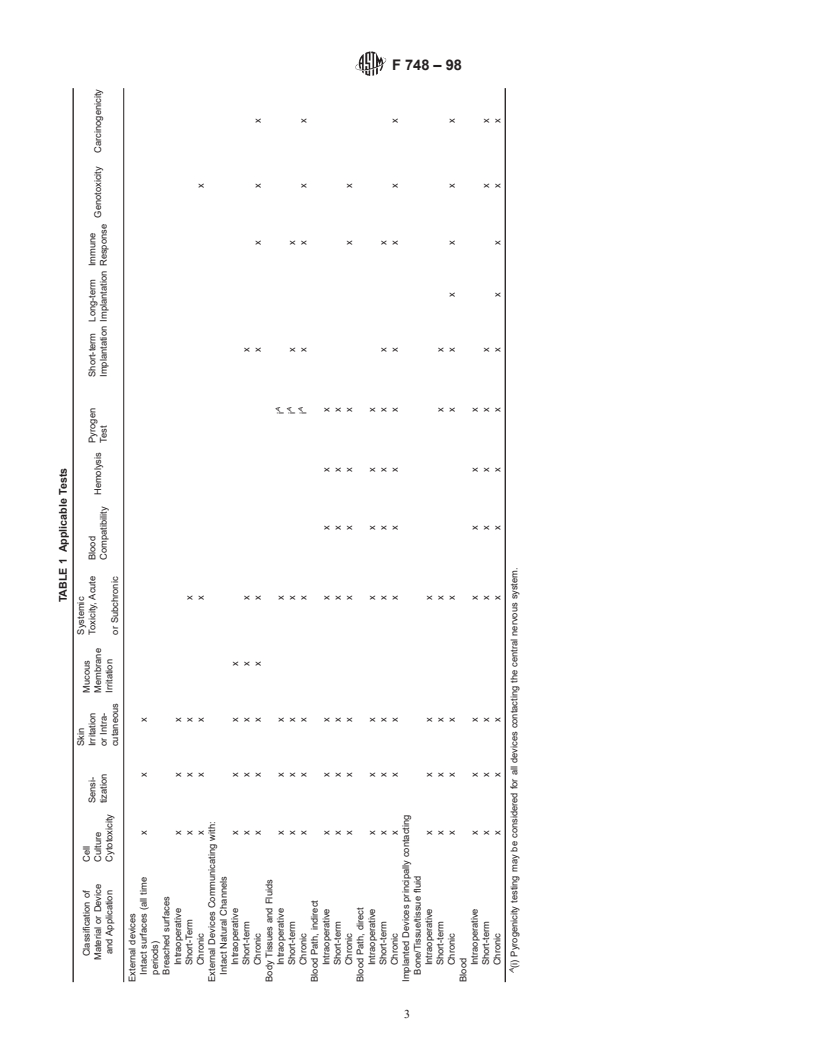
3.1 A matrix listing biological test methods versus materials candidate material failing final biological tests after extensive
(devices) and their applications is included in Table 1. The time and effort, it is not a required procedure. The investigator
expected duration of use of the device is also considered. should be aware that, should an adverse tissue response be
Intra-operative is less than 24 h, short-term is up to and observed with a final product, it may be impossible to
including 30 days, chronic is greater than 30 days. The position determine which component or process is responsible without
of row and column intersection is marked to indicate whether these initial screening tests.
the test is recommended for a material or device for the specific
5.1.3 This practice addresses two dimensions of tissue-
application indicated. The terms relating to device or material material interactions: duration and tissue type. A third dimen-
type and application are addressed in Section 5. Discussion of
sion, which should be considered is the relative size difference
applicability, current state of the art, and rationale for indi- between the host and the material, that is, to how much
vidual test methods also appears in that section.
material surface area is the host exposed. The material surface
area to body weight ratio may become a significant factor for
4. Significance and Use
porous materials, and devices of repeated short-term applica-
4.1 The objective of this practice is to recommend sufficient tions (for example, dialysis products). While this practice does
biological testing to establish a reasonable level of confidence not address the issue of “intensity factor” of increased surface
concerning the biological response to a material or device, area, the biocompatibility testing facility personnel should
consider it in their material screening and testing protocol
while at the same time avoiding unnecessary testing.
4.2 This document is intended to provide guidance to the design.
materials investigator in selecting the proper procedures to be 5.1.4 For the purposes of this document, devices, and the
carried out for the screening of new or modified materials.
materials that comprise them, are classified as to end-use
Because each material and each implant situation involves its human application as outlined in 5.2-5.4.
own unique circumstances, these recommendations should be
5.2 External Devices:
modified as necessary and do not constitute the only testing
5.2.1 Devices That Contact Intact Body Surfaces Only—
that will be required for a material nor should these guidelines
examples include electrodes, splints, external prostheses, cer-
be interpreted as minimum requirements for any particular
tain dressings, monitors of various types, or ostomy appliances.
situation. While an attempt has been made to provide recom-
5.2.2 Devices That Contact Breached Body Surfaces—
mendation for different implant circumstances, some of the
examples include ulcer, burn, and granulation tissue dressings,
recommended testing may not be necessary or reasonable for a
or healing devices.
specific material or application.
5.3 Externally Communicating Devices:
5.3.1 Devices Communicating with Intact Natural Chan-
5. Classification of Materials and Devices by End-Use
nels:
Applications
5.3.1.1 Intraoperative (<24 hours)—examples include in-
5.1 General:
traintestinal devices (such as sigmoidoscopes, colonoscopes,
5.1.1 When new materials are sought for a medical appli-
stomach tubes, or gastroscopes), tracheal tubes, bronchoscopes
cation for use on humans, the material(s) may comprise the
and any parts of ancillary equipment that are in contact with
whole final device product, or may be one of many component
materials entering the body, and irrigation sets.
materials in the device. The first step is a thorough literature
5.3.1.2 Short-term (up to and including 30 days)—examples
search for previous use of the material or biocompatibility
include contact lenses, urinary catheters, and intravaginal
testing studies to assure that it has not been known to produce
devices.
an adverse biological response that exceeds the expected
5.3.1.3 Chronic (>30 days)—examples include urinary
benefit in the use of the device. Note that the final fabricated
catheters for chronic use and intrauterine devices.
5.3.2 Devices Communicating with Body Tissues and Flu-
ids:
Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
5.3.2.1 Intraoperative (<24 hours)—examples include hy-
4th Floor, New York, NY 10036.
Available from CDRH, Rockville, MD. podermic needles, penetrating electrodes, biopsy instruments,
F748–98
TABLE 1 Applicable Tests
Skin Systemic
Classification of Cell Mucous
Sensi- Irritation Toxicity, Acute Blood Pyrogen Short-term Long-term Immune
Material or Device Culture Membrane Hemolysis Genotoxicity Carcinogenicity
tization or Intra- Compatibility Test Implantation Implantation Response
and Application Cytotoxicity Irritation
cutaneous or Subchronic
External devices
Intact surfaces (all time xx x
periods)
Breached surfaces
Intraoperative x x x
Short-Term x x x x
Chronic x x x x x
External Devices Communicating with:
Intact Natural Channels
Intraoperative x x x x
Short-term x x x x x x
Chronic x x x x x x x x x
Body Tissues and Fluids
A
Intraoperative x x x x i
A
Short-term x x x x i xx
A
Chronic x x x x i xxx x
Blood Path, indirect
Intraoperative x x x x x x x
Short-term x x x x x x x
Chronic x x x x x x x x x
Blood Path, direct
Intraoperative x x x x x x x
Short-term x x x x x x x x x
Chronic x x x x x x x x x x x
Implanted Devices principally contacting
Bone/Tissue/tissue fluid
Intraoperative x x x x
Short-term x x x x x x
Chronic x x x x x x x x x x
Blood
Intraoperative x x x x x x x
Short-term x x x x x x x x x x
Chronic x x x x x x x x x x x x
A
(i) Pyrogenicity testing may be considered for all devices contacting the central nervous system.
F748–98
arthroscopes, laparoscopes, irrigation equipment, surgical in- is substituted for actual biocompatibility testing of the extracts,
struments, trochars, and any parts of ancillary equipment that validation procedures may be necessary to show the relative
are in contact with materials entering the body. tissue response to levels of extractable which are slightly above
5.3.2.2 Short-term (up to and including 30 days)—examples the detection limit. It is particularly appropriate that animal
include cranial calipers, perfusion apparatus, drainage appara- testing involving extracts be considered for deletion if there are
tus, stabilizing orthopedic devices, and any parts of ancillary no detectable substances being extracted.
equipment that are in contact with material entering the body.
6.2 Cell Culture Cytotoxicity Assays—This test evaluates in
5.3.2.3 Chronic (>30 days)—examples include percutane-
vitro toxicity of substrate materials to cultured cells.
ous electrodes, active penetrating electrodes, stapedectomy
6.2.1 Generally materials that do not pass the cytotoxicity
prostheses, partial and total ossicular replacement prostheses,
assays are not considered for further biocompatibility testing
or tympanoplasty ventilation tubes.
and are not used in devices for human application. Thus the
5.3.3 Blood Path, Indirect—Products contacting blood path
direct relation between results of cytotoxicity testing and
at one point for usually less than 24 hours, and serves as a
biocompatibility of materials has not been documented and
conduit for fluid entry into the vascular system. Examples
there is some controversy as to the value of the testing since
include solution administration sets, extension sets, transfer
some good materials may be excluded and some others that are
sets, or blood administration sets.
not biocompatible may pass this test. Cytotoxicity testing is
5.3.3.1 Products that are used for >24 hours or that are used
recommended as an early screening test and also to provide
repeatedly in the same patient will be considered as chronic
information that will aid in the development of cytotoxicity
usage and should undergo extended testing.
tests predictive of in vivo performance.
5.3.4 Blood, Path, Direct—Single recirculating blood expo-
6.2.2 Several different tests are included under this heading,
sure or product is in blood path generally less than 24 hours.
such as Agar Diffusion, Fluid Medium, Agar Overlay, Flask
Examples include intravenous catheters, oxygenators, extracor-
Dilution, etc. All of these tests emphasize in vitro toxicity of
poreal oxygenator tubing and accessories.
either substrate materials or extract solutions to cultured cells.
5.3.5 Blood Path, Direct, Short Term, or Chronic, or re-
Cellular damage is observed and graded. Two available ver-
peated exposure
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.