ASTM F2051-00
(Specification)Standard Specification for Implantable Saline Filled Breast Prosthesis
Standard Specification for Implantable Saline Filled Breast Prosthesis
SCOPE
1.1 This specification covers the requirements for single use saline inflatable, smooth and textured silicone shell implantable breast prostheses, intended for use in surgical reconstruction, augmentation, or replacement of the breast.
1.2 Limitations
1.2.1 This specification does not cover custom fabricated implantable breast prostheses.
1.2.2 This specification does not cover gel/saline type implants, which are within the scope of F703 (Standard Specification for Implantable Breast Prostheses).
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F 2051 – 00
Standard Specification for
Implantable Saline Filled Breast Prosthesis
This standard is issued under the fixed designation F 2051; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope ANSI/AAMI/ISO 10993-1, Biological Testing of Medical
and Dental Materials and Devices – Part 1: Guidance on
1.1 This specification covers the requirements for single use
Selection of Tests
saline inflatable, smooth and textured silicone shell implant-
ANSI/AAMI/ST50-1995, Dry Heat (Heated Air) Sterilizers
able breast prostheses, intended for use in surgical reconstruc-
ANSI/AAMI/ISO 111355-1994, Medical Devices – Valida-
tion, augmentation, or replacement of the breast.
tion and Routine Control of Ethylene Oxide Sterilization
1.2 Limitations
ANSI/AAMI/ISO 11137-1994, Sterilization of Health Care
1.2.1 This specification does not cover custom fabricated
Products – Requirements for Validation and Routine and
implantable breast prostheses.
Routine Control – Radiation Sterilization
1.2.2 This specification does not cover gel/saline type im-
ANSI/AAMI/ISO 11134-1993, Sterilization of Health Care
plants, which are within the scope of F 703 (Standard Speci-
Products – Requirements for Validation and Routine
fication for Implantable Breast Prostheses).
Control – Industrial Moist Heat Sterilization
1.3 This standard does not purport to address all of the
Parenteral Drug Association, 1981 Technical Report No. 3,
safety concerns, if any, associated with its use. It is the
Validation of Dry Heat Processes Used for Sterilization
responsibility of the user of this standard to establish appro-
and Depyrogenation
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use.
3. Terminology
2. Referenced Documents 3.1 Definitions:
3.1.1 fused or adhered joints (seams)—sites in the shell or
2.1 ASTM Standards:
other parts of implantable breast prosthesis where materials
D 412 Test Methods For Rubber Properties in Tension
have been joined (fused or bonded) together, with or without
D 1349 Recommended Practices for Rubber-Standard Tem-
2 adhesive, as part of the manufacturing process.
peratures and Atmospheres for Testing and Conditioning
3.1.2 inflatable breast prosthesis—implantable breast pros-
D 3389 Standard Test Method for Coated Fabrics Abrasion
3 theses not containing silicone gel – implantable breast pros-
Resistance (Rotary Platform, Double-Head Abrader)
thesesdesignedandprovidedprefilledwithsalineoremptyand
F 604 Specification for Silicone Elastomers Used in Medi-
4 to be filled with saline at the time of use to adjust the volume
cal Applications
of the prosthesis.
F 748 Practice for Selecting Generic Biological Test Meth-
4 3.1.2.1 type 1—fixed volume inflatable breast prosthesis –
ods for Materials and Devices
an implantable breast prosthesis composed of a single lumen,
F 1251 Standard Terminology Relating to Polymeric Bio-
4 empty when supplied and having a valve to facilitate filling the
materials in Medical and Surgical Devices
lumen with saline at the time of use.
2.2 Other Documents:
5 3.1.2.2 type 2—variable volume inflatable breast prosthesis
USP (United States Pharmacopeia)
– an implantable breast prosthesis composed of a single lumen,
Federal Register, Title 21, Part 820
empty when supplied and having a valve to facilitate filling the
Association for the Advance of Medical Instrumentation
lumen with a portion of the volume of saline at the time of use.
Thevalvesystemisdesignedtofacilitatefurtherpost-operative
This specification is under the jurisdiction of ASTM Committee F04 on
adjustment with saline as instructed in product literature.
Medical and Surgical Materials and Devices and is the direct responsibility of
3.1.2.3 type 3—fixed volume inflatable breast prosthesis –
Subcommittee F04.32 on Plastic and Reconstructive Surgery.
an implantable breast prosthesis composed of a single lumen,
Current edition approved July 10, 2000. Published April 2001.
prefilled with saline by the manufacturer prior to time of use.
Annual Book of ASTM Standards, Vol 09.01.
Annual Book of ASTM Standards, Vol 09.02.
3.1.3 lumen—a cavity within a shell of an implantable
Annual Book of ASTM Standards, Vol 13.01.
breast prosthesis. Inflatable lumens are accessible by valve to
United States Pharmacopeia, Vol XXI, Mack Publishing Company, Easton, PA
facilitate the addition of saline to adjust the volume of the
1989. Available from Pharmacopeia Convention, Inc., 12601 Twinbrook Parkway,
Rockville, NC 00852. prosthesis at the time of use.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F2051–00
3.1.4 orientation means—any mark or palpable portion of shall be adequate to drive the chemistry of vulcanization of all
an implantable breast prosthesis to assist the surgeon in elastomer to completion and remove by-products of the cure in
positioning the implant. keeping with the chemical stoichiometry of the specific cure
3.1.5 saline—only sodium chloride for injection (USP) is system (e.g., after postcure no additional vulcanization should
recommended for filling lumens of inflatable breast prosthesis. occur when heated additionally at recommended cure tempera-
3.1.6 shell—a silicone elastomer continuous layer or mem- ture).
branecontainer(sac)whichenclosesalumenofanimplantable 5.1.4 Physical Property Testing and Requirements—
breast prosthesis. Silicone elastomer shells shall demonstrate an acceptable
3.1.7 silicone elastomer—an elastomer containing cross- response in physical property tests. Prostheses for testing
linked silicone polymer and fumed amorphous (non- should be selected from standard production batches which
crystalline) silica as a reinforcing filler. have gone through all manufacturing processes, including
3.1.8 valve—sealableorselfsealingopeninginaninflatable sterilization.
prosthesis, extending from the exterior surface of the shell into 5.1.4.1 Specimen Preparation—Cut required tests speci-
a lumen, designed to facilitate addition of saline at the time of mens from shells with D 412 Dies. Devices or specimens shall
use or postoperatively to adjust prosthesis volume. be conditioned before testing for at least1hat23 6 2°C (73.4
3.1.9 patch—apieceofsiliconeelastomerwhichcoversand 6 3.6°F).
seals the hole which results from the manufacturing process of 5.1.4.2 Dimension—The individual shape, range of volume
shell fabrication. (displacement), base size, and anterior projection are deter-
mined by the manufacturer.
4. Significance and Use
6. Volume and Dimensions
4.1 This specification contains requirements based on state-
of-art science and technology as applicable to various consid-
6.1 Volumes of Prostheses:
erations that have been identified as important to assure
6.1.1 Saline Inflatable Prostheses—The designed or mini-
reasonable safety and efficacy as it relates to the biocompat-
mum and maximum recommended volume of saline fill shall
ibility and the mechanical integrity of the device components
be listed in instructions for use.
in implantable breast prostheses.
6.2 Dimensions—The ranges of shapes, volumes, base
4.1.1 This standard specification is not intended to limit the
sizes, and anterior projections are determined by the manufac-
science and technology that may be considered and applied to
turer. Pertinent information shall be contained in the package
assure performance characteristics of subject breast prostheses
insert.
in intended applications. When new information becomes
available or changes in state-of-art science and technology 7. Fixation Sites
occur and relevance to subject prostheses has been established
7.1 Thepresenceoffixationsitesonanytypeofimplantable
by valid science, it is intended that this specification will be
breast prosthesis is optional. When used, the size and locations
revised in accordance with ASTM guidelines.
of fixation sites shall be clearly stated in instructions for use.
5. Materials
8. Orientation Means
5.1 Silicone Elastomer—Select and specify elastomers for
8.1 Orientation means are optional features of subject pros-
use in implantable breast prostheses in keeping with F 604.
theses. When orientation means are claimed, the location and
5.1.1 Shell—The following describes suitable silicone elas-
recommended techniques for use shall be clearly described in
tomer compositions for use as the primary material of con-
instructions for use.
struction of the shell including the exterior (tissue contact)
surface:
9. Test Methods and Requirements
polymer types MQ or VMQ
9.1 Biocompatibility:
fillers A, B or C
additive J (for radiopacity) 9.1.1 Standard Practice F 748—New or existing materials
catalysts B, G, J or K
shall be in compliance with Standard Practice F 748 or other
NOTE 1—Thecompositionlistedinthissectionarenotintendedtolimit accepted standards such as ISO/AAMI/ANSI 10993-1.Assays
the compositions that may be used providing all other requirements of this
recommended by Standard Practice F 748 include Cell Culture
specification are satisfied.
Cytotoxicity Assays, Short-Term Intramuscular Implantation
5.1.2 Fabrication—Fabrication techniques must necessarily Assay, Short-Term Subcutaneous Assay, Carcinogenicity,
be dependent on the type of elastomer, the portion of an Long-Term Implant Test, Systemic Injection (Acute Toxicity)
implantable breast prosthesis fabricated, its shape, location and Assay, Sensitization Assay, Mutagenicity, and Pyrogenicity.
function on the prosthesis. 9.1.2 Silicone Saline Filled Prostheses—Testspecimensfor
5.1.3 Vulcanization and Postcure—Timeandtemperatureof chronic implantation assays (carcnogenciity and long term
vulcanizationandpostcuremustbeadjustedwithconsideration implant tests) shall be fabricated from the same combination of
of the elastomer type and the multi-step fabrication require- silicone elastomer and by the same or similar procedures and
ments of specific prostheses. Final postcure is typically done conditions used in fabricating prostheses. The thickness of
only after the shell or shells and all other portions have been shell in specimens shall be typical of thickness used in
completely assembled. Time and temperature of final postcure prostheses.
F2051–00
9.1.3 Prior Biocompatibility Assays— When prior biocom- 9.2.3.4 Fused or Adhered Joined—Requirements for ad-
patibility data are available for silicone elastomer in clinical hered or fused silicone rubber materials shall be critical to their
use in breast implants, even if not done by the exact protocols integrity.
described in more standards, such data may satisfy all or part
9.2.3.4.1 Critical Fused orAdhered Joints— Joints or seams
of the specific biocompatibility requirements of F 748 or
that are critical to the integrity of the prostheses envelope shall
equivalent methodology.
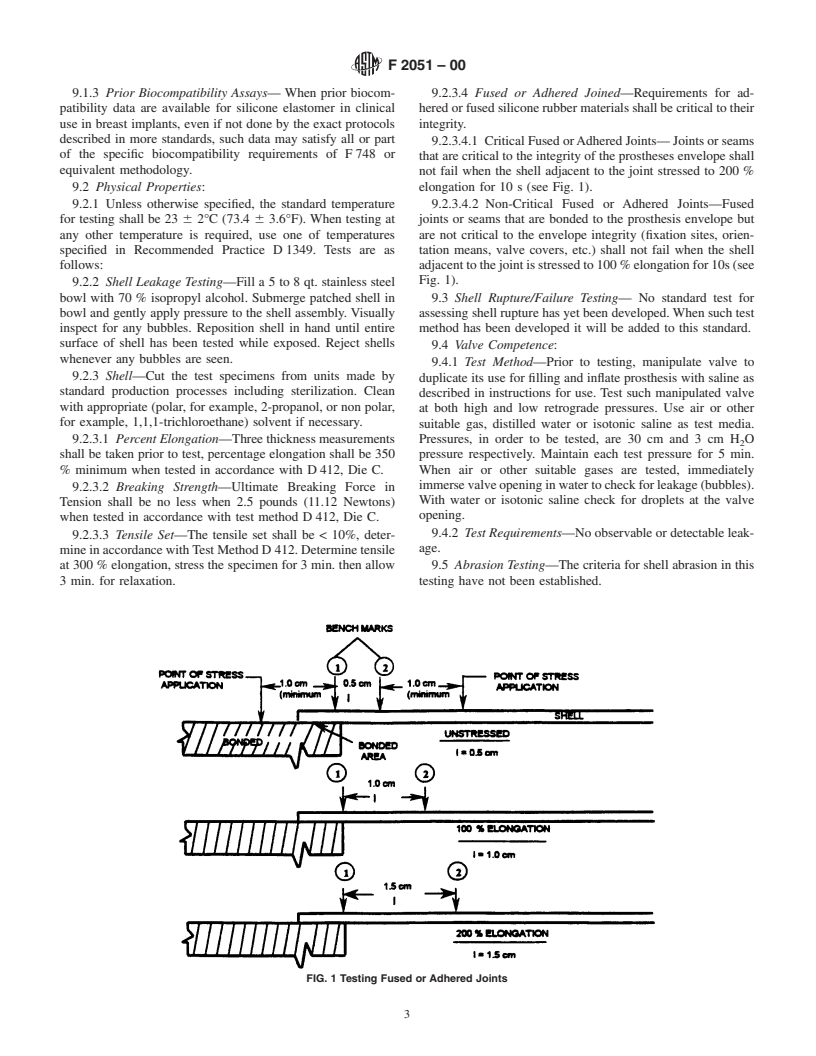
not fail when the shell adjacent to the joint stressed to 200 %
9.2 Physical Properties: elongation for 10 s (see Fig. 1).
9.2.1 Unless otherwise specified, the standard temperature 9.2.3.4.2 Non-Critical Fused or Adhered Joints—Fused
for testing shall be 23 6 2°C (73.4 6 3.6°F). When testing at joints or seams that are bonded to the prosthesis envelope but
any other temperature is required, use one of temperatures are not critical to the envelope integrity (fixation sites, orien-
specified in Recommended Practice D 1349. Tests are as tation means, valve covers, etc.) shall not fail when the shell
follows: adjacenttothejointisstressedto100%elongationfor10s(see
Fig. 1).
9.2.2 Shell Leakage Testing—Filla5to8qt. stainless steel
bowl with 70 % isopropyl alcohol. Submerge patched shell in 9.3 Shell Rupture/Failure Testing— No standard test for
bowl and gently apply pressure to the shell assembly. Visually assessing shell rupture has yet been developed. When such test
inspect for any bubbles. Reposition shell in hand until entire method has been developed it will be added to this standard.
surface of shell has been tested while exposed. Reject shells
9.4 Valve Competence:
whenever any bubbles are seen.
9.4.1 Test Method—Prior to testing, manipulate valve to
9.2.3 Shell—Cut the test specimens from units made by
duplicate its use for filling and inflate prosthesis with saline as
standard production processes including sterilization. Clean
described in instructions for use. Test such manipulated valve
with appropriate (polar, for example, 2-propanol, or non polar,
at both high and low retrograde pressures. Use air or other
for example, 1,1,1-trichloroethane) solvent if necessary.
suitable gas, distilled water or isotonic saline as test media.
9.2.3.1 Percent Elongation—Three thickness measurements Pressures, in order to be tested, are 30 cm and 3 cm H O
shall be taken prior to test, percentage elongation shall be 350 pressure respectively. Maintain each test pressure for 5 min.
% minimum when tested in accordance with D 412, Die C. When air or other suitable gases are tested, immediately
immersevalveopeninginwatertocheckforleakage(bubbles).
9.2.3.2 Breaking Strength—Ultimate Breaking Force in
With water or isotonic saline check for droplets at the valve
Tension shall be no less when 2.5 pounds (11.12 Newtons)
opening.
when tested in accordance with test method D 412, Die C.
9.4.2 Test Requirements—No observable or detectable leak-
9.2.3.3 Tensile Set—The tensile set shall be < 10%, deter-
age.
mineinaccordancewithTestMethodD 412.Determinetensile
9.5 Abrasion Testing—The criteria for shell abrasion in this
at 300 % elongation, stress the specimen for 3 min. then allow
3 min. for relaxation. testing have not been established.
FIG. 1 Testing Fused or Adhered Joints
F2051–00
9.5.1 Abrasion Testing—Wet method – See A1.1. 11.2.1 Manufacturer’s name and address.
9.5.2 Abrasion Testing—Dry method – See A1.2. 11.2.2 Product name, shape, type and lot number.
9.5.3 Particle sizes generated by these test methods may not 11.2.3 Minimum and maximum volume and relevant di-
beabletobecorrelatedwithparticulatesresultingfromclinical mension information.
use, and therefore, has questionable meaning. 11.2.4 Date (month and year) of sterilization or packaging
and method of sterilization.
10. Sterilization
11.2.5 Special storage requirements, if any.
10.1 Implantable breast prostheses may be supplied pre- 11.2.6 Self-adhering label suitable for application to the
sterilized in accordance with currentAMI and PDAprocedures patient’s medical records containing follo
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.