ASTM E1232-07(2019)
(Test Method)Standard Test Method for Temperature Limit of Flammability of Chemicals
Standard Test Method for Temperature Limit of Flammability of Chemicals
SIGNIFICANCE AND USE
5.1 The lower temperature limit of flammability is the minimum temperature at which a liquid (or solid) chemical will evolve sufficient vapors to form a flammable mixture with air under equilibrium conditions. Knowledge of this temperature is important in determining guidelines for the safe handling of chemicals, particularly in closed process and storage vessels.
Note 1: As a result of physical factors inherent in flash point apparatus and procedures, closed-cup flash point temperatures are not necessarily the minimum temperature at which a chemical will evolve flammable vapors (see Appendix X2 and Appendix X3, taken in part from Test Method E502). The temperature limit of flammability test is designed to supplement limitations inherent in flash point tests (Appendix X2). It yields a result closely approaching the minimum temperature of flammable vapor formation for equilibrium situations in the chemical processing industry such as in closed process and storage vessels.
Note 2: As a result of flame quenching effects existing when testing in standard closed-cup flash point apparatus, there are certain chemicals that exhibit no flash point but do evolve vapors that will propagate a flame in vessels of adequate size (X3.2). The temperature limit of flammability test chamber is sufficiently large to overcome flame quenching effects in most cases of practical importance, thus, usually indicating the presence of vapor-phase flammability if it does exist (6.2).
Note 3: The lower temperature limit of flammability (LTL) is only one of several characteristics that should be evaluated to determine the safety of a specific material for a specific application. For example, some materials are found to have an LTL by this test method when, in fact, other characteristics such as minimum ignition energy and heat of combustion should also be considered in an overall flammability evaluation.
5.2 The vapor concentration present at the lower temperature limit of flammability e...
SCOPE
1.1 This test method covers the determination of the minimum temperature at which vapors in equilibrium with a liquid (or solid) chemical will be sufficiently concentrated to form flammable mixtures in air at atmospheric pressure. This test method is written specifically for determination of the temperature limit of flammability of systems using air as the source of oxidant and diluent. It may also be used for other oxidant/diluent combinations, including air plus diluent mixtures; however, no oxidant/diluent combination stronger than air should be used. Also, no unstable chemical capable of explosive decomposition reactions should be tested (see 8.3).
1.2 This test method is designed and written to be run at local ambient pressure and is limited to a maximum initial pressure of 1 atm abs. It may also be used for reduced pressures with the practical lower pressure limit being approximately 13.3 kPa (100 mm Hg). The maximum practical operating temperature of this equipment is approximately 150°C (302°F) (Note A1.2).
1.3 The values stated in SI units are to be regarded as standard. The values given in parentheses are mathematical conversions to inch-pound units that are provided for information only and are not considered standard.
1.4 This standard should be used to measure and describe the properties of materials, products, or assemblies in response to heat and flame under controlled laboratory conditions, and should not be used to describe or appraise the fire hazard or fire risk of materials, products, or assemblies under actual fire conditions. However, results of this test may be used as elements of a fire risk assessment which takes into account all of the factors which are pertinent to an assessment of the fire hazard of a particular end use.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to...
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E1232 − 07 (Reapproved 2019)
Standard Test Method for
Temperature Limit of Flammability of Chemicals
This standard is issued under the fixed designation E1232; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
INTRODUCTION
The temperature limit of flammability test measures the minimum temperature at which liquid (or
solid) chemicals evolve sufficient vapors to form a flammable mixture with air under equilibrium
conditions. This temperature is applicable for assessing flammability in large process vessels and
similar equipment (Appendix X1 and Appendix X2).
1. Scope elements of a fire risk assessment which takes into account all
of the factors which are pertinent to an assessment of the fire
1.1 This test method covers the determination of the mini-
hazard of a particular end use.
mum temperature at which vapors in equilibrium with a liquid
1.5 This standard does not purport to address all of the
(or solid) chemical will be sufficiently concentrated to form
safety concerns, if any, associated with its use. It is the
flammable mixtures in air at atmospheric pressure. This test
responsibility of the user of this standard to establish appro-
methodiswrittenspecificallyfordeterminationofthetempera-
priate safety, health, and environmental practices and deter-
ture limit of flammability of systems using air as the source of
mine the applicability of regulatory limitations prior to use.
oxidant and diluent. It may also be used for other oxidant/
Specific safety precautions are given in Section 8.
diluent combinations, including air plus diluent mixtures;
1.6 This international standard was developed in accor-
however, no oxidant/diluent combination stronger than air
dance with internationally recognized principles on standard-
should be used. Also, no unstable chemical capable of explo-
ization established in the Decision on Principles for the
sive decomposition reactions should be tested (see 8.3).
Development of International Standards, Guides and Recom-
1.2 This test method is designed and written to be run at
mendations issued by the World Trade Organization Technical
local ambient pressure and is limited to a maximum initial
Barriers to Trade (TBT) Committee.
pressureof1atmabs.Itmayalsobeusedforreducedpressures
with the practical lower pressure limit being approximately
2. Referenced Documents
13.3 kPa (100 mm Hg). The maximum practical operating
2.1 ASTM Standards:
temperature of this equipment is approximately 150°C (302°F)
(Note A1.2). D3278 Test Methods for Flash Point of Liquids by Small
Scale Closed-Cup Apparatus
1.3 The values stated in SI units are to be regarded as
D3828 Test Methods for Flash Point by Small Scale Closed
standard. The values given in parentheses are mathematical
Cup Tester
conversions to inch-pound units that are provided for informa-
D3941 Test Method for Flash Point by the Equilibrium
tion only and are not considered standard.
Method With a Closed-Cup Apparatus
1.4 This standard should be used to measure and describe
E220 Test Method for Calibration of Thermocouples By
the properties of materials, products, or assemblies in response
Comparison Techniques
to heat and flame under controlled laboratory conditions, and
E230 Specification for Temperature-Electromotive Force
shouldnotbeusedtodescribeorappraisethefirehazardorfire
(emf) Tables for Standardized Thermocouples
risk of materials, products, or assemblies under actual fire
E502 Test Method for Selection and Use of ASTM Stan-
conditions. However, results of this test may be used as
dards for the Determination of Flash Point of Chemicals
by Closed Cup Methods
This test method is under the jurisdiction ofASTM Committee E27 on Hazard
Potential of Chemicals and is the direct responsibility of Subcommittee E27.04 on
Flammability and Ignitability of Chemicals. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved Feb. 1, 2019. Published March 2019. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 1991. Last previous edition approved in 2013 as E1232 – 07 (2013). Standards volume information, refer to the standard’s Document Summary page on
DOI: 10.1520/E1232-07R19. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E1232 − 07 (2019)
and procedures, closed-cup flash point temperatures are not necessarily
E537 Test Method for The Thermal Stability of Chemicals
the minimum temperature at which a chemical will evolve flammable
by Differential Scanning Calorimetry
vapors (see Appendix X2 and Appendix X3, taken in part from Test
E681 Test Method for Concentration Limits of Flammability
Method E502). The temperature limit of flammability test is designed to
of Chemicals (Vapors and Gases)
supplement limitations inherent in flash point tests (Appendix X2). It
E698 Test Method for Kinetic Parameters for Thermally
yields a result closely approaching the minimum temperature of flam-
mable vapor formation for equilibrium situations in the chemical process-
Unstable Materials Using Differential Scanning Calorim-
ing industry such as in closed process and storage vessels.
etry and the Flynn/Wall/Ozawa Method
NOTE2—Asaresultofflamequenchingeffectsexistingwhentestingin
2.2 ANSI Standard:
standard closed-cup flash point apparatus, there are certain chemicals that
ANSI-MC96.1 Temperature Measurement Thermocouples
exhibit no flash point but do evolve vapors that will propagate a flame in
vessels of adequate size (X3.2).The temperature limit of flammability test
2.3 NFPA Standard:
chamber is sufficiently large to overcome flame quenching effects in most
NFPA 325 Fire Hazardous Properties Liquids
cases of practical importance, thus, usually indicating the presence of
vapor-phase flammability if it does exist (6.2).
3. Terminology
NOTE3—Thelowertemperaturelimitofflammability(LTL)isonlyone
of several characteristics that should be evaluated to determine the safety
3.1 Definitions:
of a specific material for a specific application. For example, some
3.1.1 flash point, n—the lowest temperature, corrected to a
materialsarefoundtohaveanLTLbythistestmethodwhen,infact,other
pressure of 101.3 kPa (760 mm Hg, 1013 mbar), at which
characteristics such as minimum ignition energy and heat of combustion
application of an ignition source causes the vapors of the
should also be considered in an overall flammability evaluation.
specimen to ignite under specified conditions of test.
5.2 The vapor concentration present at the lower tempera-
3.1.2 lower limit of flammability or lower flammable limit,
ture limit of flammability equals the lower flammable limit
(LFL), n—the minimum concentration of a combustible sub-
concentration as measured by Test Method E681 and extrapo-
stance that is capable of propagating a flame through a
lated back to the same temperature. (This permits estimation of
homogeneous mixture of the combustible and a gaseous
lower temperature limits of flammability if vapor pressure and
oxidizer under the specified conditions of test.
concentrationlimitofflammabilitydataareavailable(A2.3).A
3.1.3 lower temperature limit of flammability, (LTL), n—the comparison of results of the tests, thus, affords a check on test
lowest temperature, corrected to a pressure of 101.3 kPa (760
reliability, the reliability of vapor pressure data, or both.)
mm Hg, 1013 mbar), at which application of an ignition source
causes a homogeneous mixture of a gaseous oxidizer and
6. Interferences
vaporsinequilibriumwithaliquid(orsolid)specimentoignite
6.1 This test method is not applicable to materials that
and propagate a flame away from the ignition source under the
undergo chemical changes when mixed with air. Examples
specified conditions of test.
include, but are not limited to, oxidation and polymerization.
3.2 Definitions of Terms Specific to This Standard:
6.2 Measured temperature limits are influenced by flame
3.2.1 propagation of flame, n—the upward and outward
quenching effects of the test vessel walls. The test vessel
movement of the flame front from the ignition source to the
employed in this test method is of sufficient size to eliminate
vessel walls, that is determined by visual observation.
these effects for most materials. For certain amines, haloge-
nated materials, etc., that have large ignition-quenching
4. Summary of Test Method
distances, tests should be conducted in vessels with larger
4.1 A pool of liquid is stirred in a closed vessel in an air
diameters than the one listed in this test method (A1.1).
atmosphere.The vapor-air mixture above this liquid is exposed
Quenching effects become increasingly significant as the test
to an ignition source and the upward and outward propagation
pressure decreases.
of flame away from the ignition source is noted by visual
6.3 Measured temperature limits of flammability of chemi-
observation. Temperature in the test vessel is varied between
cals can be greatly influenced, as are flash points, by the
trials until the minimum temperature at which flame will
presence of various impurities or known mixture components.
propagate away from the ignition source is determined.
Small quantities of volatile flammable impurities can reduce
5. Significance and Use
temperature limit values, and volatile inert diluents can raise
temperature limit values or produce complete inerting. (See
5.1 The lower temperature limit of flammability is the
8.2.3 and Annex A3 for a discussion of mixture testing.)
minimumtemperatureatwhichaliquid(orsolid)chemicalwill
evolve sufficient vapors to form a flammable mixture with air
7. Apparatus
under equilibrium conditions. Knowledge of this temperature
is important in determining guidelines for the safe handling of
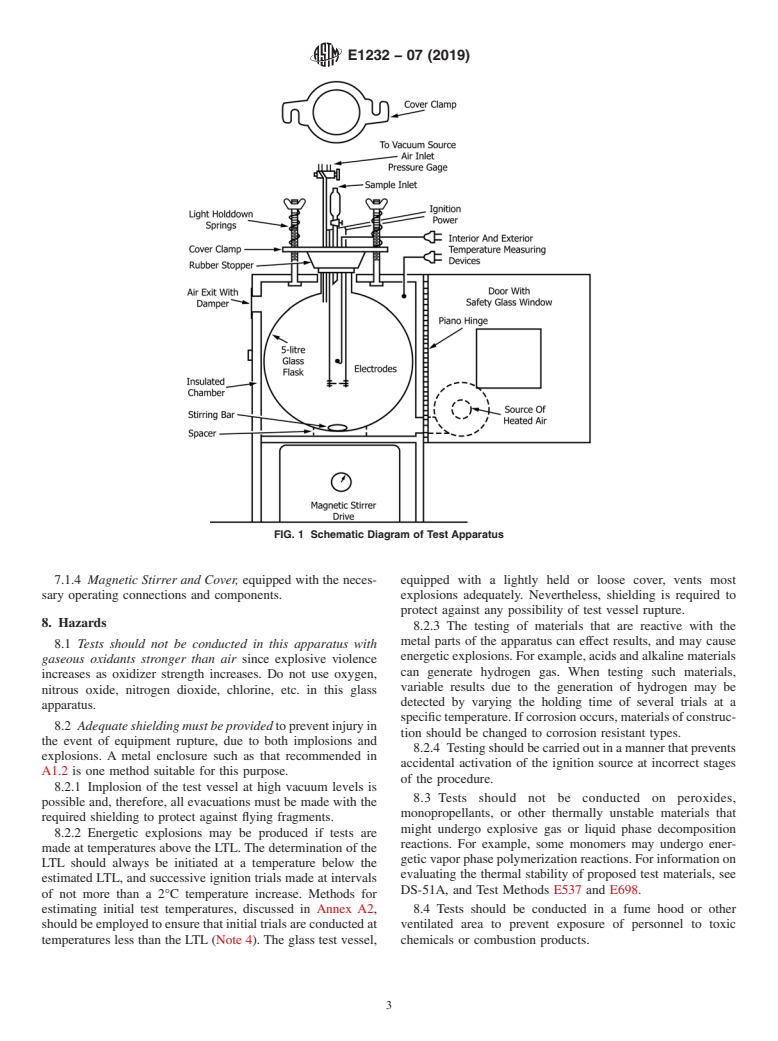
7.1 Fig. 1 is a schematic diagram of the apparatus; details
chemicals, particularly in closed process and storage vessels.
and dimensions are presented in Annex A1. The apparatus
consists of the following:
NOTE1—Asaresultofphysicalfactorsinherentinflashpointapparatus
7.1.1 Glass Test Vessel,
7.1.2 Insulated Chamber, equipped with a source of
Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
controlled-temperature air,
4th Floor, New York, NY 10036, http://www.ansi.org.
7.1.3 Ignition Device, with an appropriate power supply,
Available from National Fire Protection Association (NFPA), 1 Batterymarch
Park, Quincy, MA 02169-7471, http://www.nfpa.org. and
E1232 − 07 (2019)
FIG. 1 Schematic Diagram of Test Apparatus
7.1.4 Magnetic Stirrer and Cover, equipped with the neces- equipped with a lightly held or loose cover, vents most
sary operating connections and components. explosions adequately. Nevertheless, shielding is required to
protect against any possibility of test vessel rupture.
8. Hazards
8.2.3 The testing of materials that are reactive with the
metal parts of the apparatus can effect results, and may cause
8.1 Tests should not be conducted in this apparatus with
energeticexplosions.Forexample,acidsandalkalinematerials
gaseous oxidants stronger than air since explosive violence
can generate hydrogen gas. When testing such materials,
increases as oxidizer strength increases. Do not use oxygen,
variable results due to the generation of hydrogen may be
nitrous oxide, nitrogen dioxide, chlorine, etc. in this glass
detected by varying the holding time of several trials at a
apparatus.
specifictemperature.Ifcorrosionoccurs,materialsofconstruc-
8.2 Adequateshieldingmustbeprovidedtopreventinjuryin
tion should be changed to corrosion resistant types.
the event of equipment rupture, due to both implosions and
8.2.4 Testingshouldbecarriedoutinamannerthatprevents
explosions. A metal enclosure such as that recommended in
accidental activation of the ignition source at incorrect stages
A1.2 is one method suitable for this purpose.
of the procedure.
8.2.1 Implosion of the test vessel at high vacuum levels is
8.3 Tests should not be conducted on peroxides,
possible and, therefore, all evacuations must be made with the
monopropellants, or other thermally unstable materials that
required shielding to protect against flying fragments.
might undergo explosive gas or liquid phase decomposition
8.2.2 Energetic explosions may be produced if tests are
reactions. For example, some monomers may undergo ener-
made at temperatures above the LTL. The determination of the
geticvaporphasepolymerizationreactions.Forinformationon
LTL should always be initiated at a temperature below the
evaluating the thermal stability of proposed test materials, see
estimated LTL, and successive ignition trials made at intervals
DS-51A, and Test Methods E537 and E698.
of not more than a 2°C temperature increase. Methods for
estimating initial test temperatures, discussed in Annex A2, 8.4 Tests should be conducted in a fume hood or other
shouldbeemployedtoensurethatinitialtrialsareconductedat ventilated area to prevent exposure of personnel to toxic
temperatures less than the LTL (Note 4). The glass test vessel, chemicals or combustion products.
E1232 − 07 (2019)
NOTE 6—If mixing is inadequate, vapor concentrations can vary
8.5 Precautions must be taken to ensure that the high
throughout the flask, and inconsistent results will be obtained. Some
voltage spark ignition source is always adequately insulated
regions may contain insufficient fuel to propagate a flame at temperatures
from other electrical circuits and metal parts of the apparatus,
above the true equilibrium flammable limit temperature.
fume hood, etc. to prevent electrical hazards to personnel and
10.2.5 Turn off the stirrer.
instrumentation. Careful attention to electrical insulation integ-
10.2.6 Record the test temperature and system pressure
rity plus the use of disconnection procedures are required to
(usually barometric pressure unless system is being operated at
achieve a satisfactory protection against electrical hazards.
sub-ambient pressure).
10.2.7 Disconnect instrumentation lines as required and
9. Calibration
connect the ignition wires.
9.1 System temperature and pressure and barometric pres-
10.2.8 Check for liquid condensation or mist in the vapor
sure measuring devices must be calibrated against adequate
regions of the flask. Heat, insulate, or both, to prevent
standards.Fo
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.