ASTM E537-12
(Test Method)Standard Test Method for The Thermal Stability of Chemicals by Differential Scanning Calorimetry
Standard Test Method for The Thermal Stability of Chemicals by Differential Scanning Calorimetry
SIGNIFICANCE AND USE
5.1 This test method is useful in detecting potentially hazardous reactions including those from volatile chemicals and in estimating the temperatures at which these reactions occur and their enthalpies (heats). This test method is recommended as an early test for detecting the thermal hazards of an uncharacterized chemical substance or mixture (see Section 8).
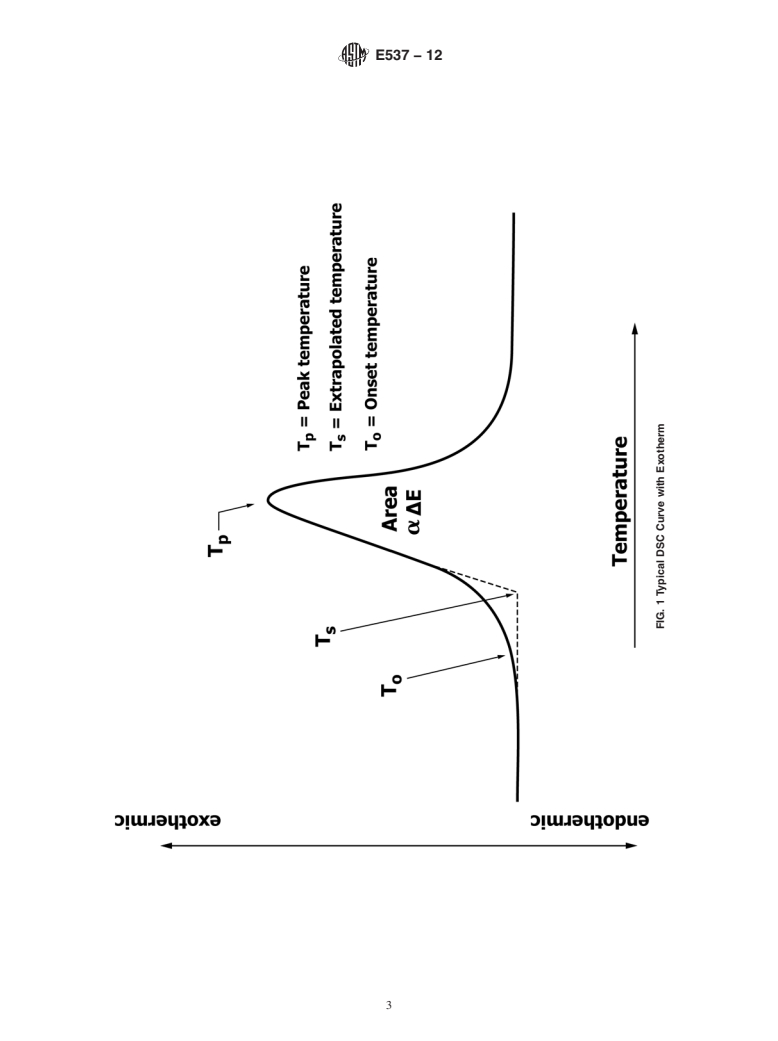
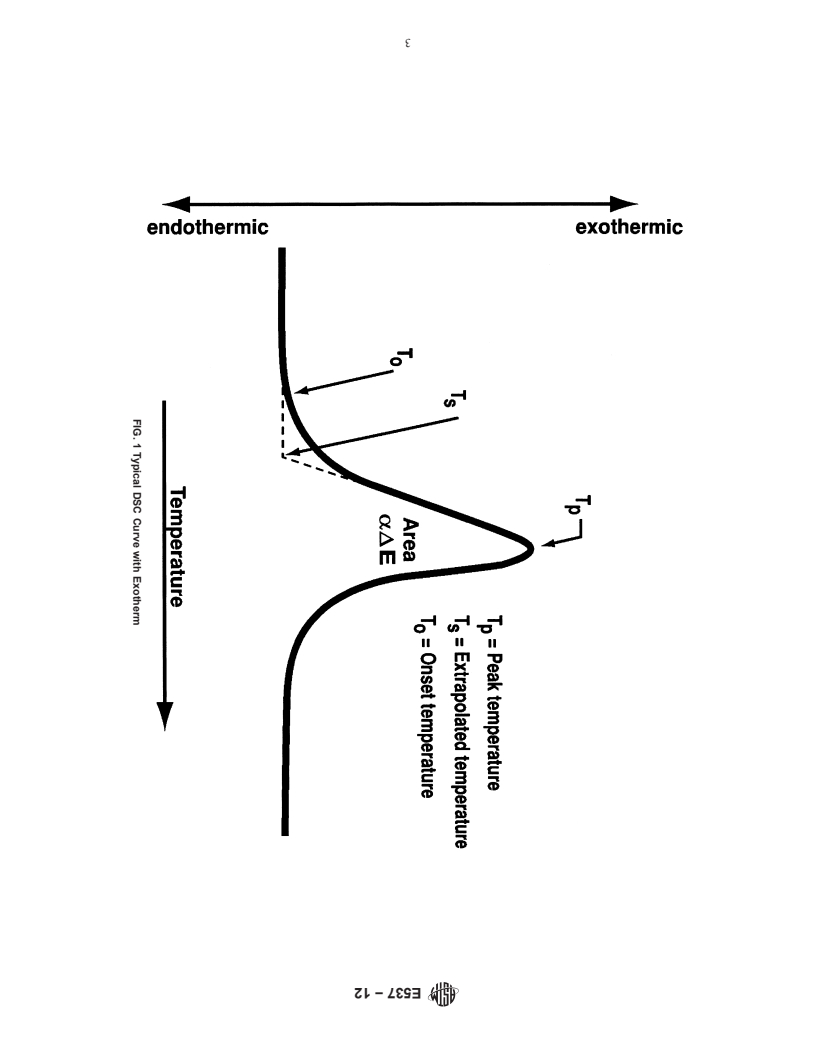
5.2 The magnitude of the change of enthalpy may not necessarily denote the relative hazard in a particular application. For example, certain exothermic reactions are often accompanied by gas evolution that increases the potential hazard. Alternatively, the extent of energy release for certain exothermic reactions may differ widely with the extent of confinement of volatile products. Thus, the presence of an exotherm and its approximate temperature are the most significant criteria in this test method (see Section 3 and Fig. 1).
5.3 When volatile substances are being studied, it is important to perform this test with a confining pressurized atmosphere so that changes of enthalpy that can occur above normal boiling or sublimation points may be detected. As an example, an absolute pressure of 1.14 MPa (150 psig) will generally elevate the boiling point of a volatile organic substance 100°C. Under these conditions exothermic decomposition is often observed.
5.4 For some substances the rate of enthalpy change during an exothermic reaction may be small at normal atmospheric pressure, making an assessment of the temperature of instability difficult. Generally a repeated analysis at an elevated pressure will improve the assessment by increasing the rate of change of enthalpy. Note 1—The choice of pressure may sometimes be estimated by the pressure of the application to which the material is exposed.
5.5 The four significant criteria of this test method are: the detection of a change of enthalpy; the approximate temperature at which the event occurs; the estimation of its enthalpy and the observance of...
SCOPE
1.1 This test method describes the ascertainment of the presence of enthalpic changes in a test specimen, using minimum quantities of material, approximates the temperature at which these enthalpic changes occur and determines their enthalpies (heats) using differential scanning calorimetry or pressure differential scanning calorimetry.
1.2 This test method may be performed on solids, liquids, or slurries.
1.3 This test method may be performed in an inert or a reactive atmosphere with an absolute pressure range from 100 Pa through 7 MPa and over a temperature range from 300 to 800 K (27 to 527°C).
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 There is no ISO standard equivalent to this test method.
1.6 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all of the safety concerns associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific safety precautions are given in Section 8.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E537 − 12
Standard Test Method for
The Thermal Stability of Chemicals by Differential Scanning
1
Calorimetry
This standard is issued under the fixed designation E537; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
INTRODUCTION
Committee E27 is currently engaged in developing methods to determine the hazard potential of
chemicals.An estimate of this potential may usually be obtained by the use of program CHETAH 7.0
2
to compute the maximum energy of reaction of the chemical or mixture of chemicals.
The expression “hazard potential” as used by this committee is defined as the degree of
susceptibility of material to ignition or release of energy under varying environmental conditions.
The primary purpose of this test method is to detect enthalpic changes and to approximate the
temperatureofinitiationandenthalpies(heats)oftheseevents.Differentialscanningcalorimetryoffers
the advantage of using very small specimens on the order of a few milligrams.
1. Scope the responsibility of the user of this standard to establish
appropriate safety and health practices and determine the
1.1 This test method describes the ascertainment of the
applicability of regulatory limitations prior to use. Specific
presence of enthalpic changes in a test specimen, using
safety precautions are given in Section 8.
minimum quantities of material, approximates the temperature
at which these enthalpic changes occur and determines their
2. Referenced Documents
enthalpies (heats) using differential scanning calorimetry or
3
2.1 ASTM Standards:
pressure differential scanning calorimetry.
E473 Terminology Relating to Thermal Analysis and Rhe-
1.2 Thistestmethodmaybeperformedonsolids,liquids,or
ology
slurries.
E691 Practice for Conducting an Interlaboratory Study to
1.3 This test method may be performed in an inert or a
Determine the Precision of a Test Method
reactive atmosphere with an absolute pressure range from 100
E967 Test Method for Temperature Calibration of Differen-
Pa through 7 MPa and over a temperature range from 300 to
tial Scanning Calorimeters and Differential Thermal Ana-
800 K (27 to 527°C).
lyzers
E968 Practice for Heat Flow Calibration of Differential
1.4 The values stated in SI units are to be regarded as
Scanning Calorimeters
standard. No other units of measurement are included in this
E1445 Terminology Relating to Hazard Potential of Chemi-
standard.
cals
1.5 There is no ISO standard equivalent to this test method.
E1860 Test Method for Elapsed Time Calibration of Ther-
1.6 This standard may involve hazardous materials,
mal Analyzers
operations, and equipment. This standard does not purport to
address all of the safety concerns associated with its use. It is
3. Terminology
3.1 Definitions:
1
This test method is under the jurisdiction ofASTM Committee E27 on Hazard
3.1.1 Specific technical terms used in this standard are
Potential of Chemicals and is the direct responsibility of Subcommittee E27.02 on
defined in Terminologies E473 and E1445, and include
Thermal Stability and Condensed Phases.
Current edition approved Dec. 1, 2012. Published December 2012. Originally
approved in 1976. Last previous edition approved in 2007 as E537 – 07. DOI:
3
10.1520/E0537-12. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
2
A complete assessment of the hazard potential of chemicals must take into contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
account a number of realistic factors not considered in this test method or the Standards volume information, refer to the standard’s Document Summary page on
CHETAH program. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E537 − 12
calorimeter, differential scanning calorimetry, extrapolated on- elevate the boiling point of a volatile organic substance 100°C.
set value, first-deviation-from baseline, peak, reaction, and Under these conditions exothermic decomposition is often
thermal stability. observed.
3.2 Definitions of Terms Specific to This Standard:
5.4 For some substances the rate of enthalpy change during
3.2.1 DSC curve—a record of a differential scanning calo-
an exothermic reaction may be small at normal atmospheric
rimeter where the change in heat flow (∆q) is plotted on the
pressure, making an assessment of the temperature of instabil-
ordinate and temperature or time is plotted on the absciss
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E537 − 07 E537 − 12
Standard Test Method for
The Thermal Stability of Chemicals by Differential Scanning
1
Calorimetry
This standard is issued under the fixed designation E537; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
INTRODUCTION
Committee E27 is currently engaged in developing methods to determine the hazard potential of
chemicals. An estimate of this potential may usually be obtained by the use of program CHETAH 7.0
2
to compute the maximum energy of reaction of the chemical or mixture of chemicals.
The expression “hazard potential” as used by this committee is defined as the degree of
susceptibility of material to ignition or release of energy under varying environmental conditions.
The primary purpose of this test method is to detect enthalpic changes and to approximate the
temperature of initiation and enthalpies (heats) of these events. Differential scanning calorimetry offers
the advantage of using very small specimens on the order of a few milligrams.
1. Scope
1.1 This test method describes the ascertainment of the presence of enthalpic changes in a test specimen, using minimum
quantities of material, approximates the temperature at which these enthalpic changes occur and determines their enthalpies (heats)
using differential scanning calorimetry or pressure differential scanning calorimetry.
1.2 This test method may be performed on solids, liquids, or slurries.
1.3 This test method may be performed in an inert or a reactive atmosphere with an absolute pressure range from 100 Pa through
7 MPa and over a temperature range from 300 to 800 K (27 to 527°C ). 527°C).
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 There is no ISO standard equivalent to this test method.
1.6 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all
of the safety concerns associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and
health practices and determine the applicability of regulatory limitations prior to use. Specific safety precautions are given in
Section 8.
2. Referenced Documents
3
2.1 ASTM Standards:
E473 Terminology Relating to Thermal Analysis and Rheology
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
E967 Test Method for Temperature Calibration of Differential Scanning Calorimeters and Differential Thermal Analyzers
E968 Practice for Heat Flow Calibration of Differential Scanning Calorimeters
E1445 Terminology Relating to Hazard Potential of Chemicals
1
This test method is under the jurisdiction of ASTM Committee E27 on Hazard Potential of Chemicals and is the direct responsibility of Subcommittee E27.02 on Thermal
Stability and Condensed Phases.
Current edition approved Oct. 1, 2007Dec. 1, 2012. Published October 2007 December 2012. Originally approved in 1976. Last previous edition approved in 20022007
as E537 – 02.E537 – 07. DOI: 10.1520/E0537-07.10.1520/E0537-12.
2
A complete assessment of the hazard potential of chemicals must take into account a number of realistic factors not considered in this test method or the CHETAH
program.
3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E537 − 12
E1860 Test Method for Elapsed Time Calibration of Thermal Analyzers
3. Terminology
3.1 Definitions:
3.1.1 Specific technical terms used in this standard are defined in Terminologies E473 and E1445. , and include calorimeter,
differential scanning calorimetry, extrapolated onset value, first-deviation-from baseline, peak, reaction, and thermal stability.
3.2 Definitions of Terms Specific to This Standard:
3.2.1 DSC curve—a record of a differential scanning calorimeter where the change in heat flow (Δq) is plotted on the ordinate
a
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.