ASTM F451-99a
(Specification)Standard Specification for Acrylic Bone Cement
Standard Specification for Acrylic Bone Cement
SCOPE
1.1 This specification covers self-curing resins used primarily for the fixation of internal orthopedic prostheses. The mixture may be used in either the predough or dough stage in accordance with the manufacturer's recommendations.
1.2 Units of premeasured powder and liquid are supplied in a form suitable for mixing, which then sets in place.
1.3 While a variety of copolymers and comonomers may be incorporated, the composition of the set cement shall contain poly(methacrylic acid esters) as its main ingredient.
1.4 This specification covers compositional, physical performance, and biocompatibility as well as packaging requirements. The biocompatibility of acrylic bone cement as it has been traditionally formulated and used has been reported in the literature (1, 2).
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 451 – 99a
Standard Specification for
Acrylic Bone Cement
This standard is issued under the fixed designation F 451; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope ods for Materials and Devices
F 749 Practice for Evaluating Material Extracts by Intracu-
1.1 This specification covers self-curing resins used prima-
taneous Injection in the Rabbit
rily for the fixation of internal orthopedic prostheses. The
F 756 Practice for Assessment of Hemolytic Properties of
mixture may be used in either the predough or dough stage in
Materials
accordance with the manufacturer’s recommendations.
F 763 Practice for Short-Term Screening of Implant Mate-
1.2 Units of premeasured powder and liquid are supplied in
rials
a form suitable for mixing. The mixture then sets in place.
F 813 Practice for Direct Contact Cell Culture Evaluation of
1.3 While a variety of copolymers and comonomers may be
Materials for Medical Devices
incorporated, the composition of the set cement shall contain
F 895 Practice for Agar Diffusion Cell Culture Screening
poly(methacrylic acid esters) as its main ingredient.
for Cytotoxicity
1.4 This specification covers compositional, physical per-
F 981 Practice for Assessment of Compatibility of Bioma-
formance, and biocompatibility as well as packaging require-
terials (Nonporous) for Surgical Implants with Respect to
ments. The biocompatibility of acrylic bone cement as it has
Effect of Materials on Muscle and Bone
been traditionally formulated and used has been reported in the
2.2 ANSI/ADA Standard:
literature (1, 2).
No. 15 Specification for Acrylic Resin Teeth
1.5 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
3. Terminology
responsibility of the user of this standard to establish appro-
3.1 Definitions of Terms Specific to This Standard:
priate safety and health practices and determine the applica-
3.1.1 doughing time—the time after commencement of
bility of regulatory limitations prior to use.
mixing at which the mixture ceases to adhere to a standard
2. Referenced Documents probe (see 7.5).
3.1.2 exothermic or maximum temperature—the maximum
2.1 ASTM Standards:
temperature of the mixture due to self-curing in a standard
D 695 Test Method for Compressive Properties of Rigid
mold (see 7.6).
Plastics
3.1.3 extrusion—the rate of flow of the material through a
D 3835 Test Method for Determination of Properties of
standard orifice under load (see 7.8.1).
Polymeric Materials by Means of a Capillary Rheometer
3.1.4 intrusion—the distance of flow of the mixture into a
E 29 Practice for Using Significant Digits in Test Data to
standard mold under load (see 7.8.2).
Determine Conformance With Specifications
3.1.5 setting time—the time after commencement of mixing
E 141 Practice for Acceptance of Evidence Based on the
at which the temperature of the curing mass equals the average
Results of Probability Sampling
6 of the maximum and ambient temperatures (see 7.7).
F 619 Practice for Extraction of Medical Plastics
3.1.6 unit—one package or vial of premeasured powder
F 748 Practice for Selecting Generic Biological Test Meth-
component and one package or vial of premeasured liquid
component.
This specification is under the jurisdiction of ASTM Committee F-4 on Medical
4. Physical Requirements
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.11 on Polymeric Materials.
4.1 Liquid:
Current edition approved May 10, 1999. Published July 1999. Originally
4.1.1 Appearance—The liquid shall be free of extraneous
published as F 451 – 76. Last previous edition F 451 – 95.
particulate matter or obvious visual contaminants in its con-
The boldface numbers in parentheses refer to the list of references at the end of
this standard. tainer.
Annual Book of ASTM Standards, Vol 08.01.
Annual Book of ASTM Standards, Vol 08.02.
5 7
Annual Book of ASTM Standards, Vol 14.02. Available from American National Standards Institute, 11 W. 42nd St., 13th
Annual Book of ASTM Standards, Vol 13.01. Floor, New York, NY 10036.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F 451 – 99a
TABLE 2 Requirements for Cured Polymer After Setting
4.1.2 Stability—After being heated for 48 h at 60 6 2°C, the
viscosity of the liquid shall not increase by more than 10 % of Property Requirement
its original value (see 7.3).
Compressive Strength, min., MPa 70
4.1.3 Sterility—The liquid, as poured from its container,
shall pass the tests described in “Sterility Tests—Liquid and
Ointments” (7.4) (3).
7.2 Inspection—Use visual inspection in determining com-
4.2 Powder:
pliance to the requirements outlined in 4.1.1, 4.2.1, 8.1 and 8.2.
4.2.1 Appearance—The powder shall be pourable and free
7.2.1 The liquid component of two separate units shall
of extraneous materials, such as dirt or lint (7.2.2).
comply with the requirements of 4.1.1 and 8.1.
4.2.2 Sterility—The powder, as poured from its package,
7.2.2 The powder component of two separate units shall
shall pass the tests described in “Sterility Tests—Solids” (7.4)
comply with the requirements of 4.2.1 and 8.1.
(2).
7.3 Liquid Component Viscosity—Record the viscosity
4.3 Powder-Liquid Mixture:
change of two separate units (4.1.2) before and after the
4.3.1 If the mixture is to be used in its predough stage, the
heating exposure by timing the flow of the liquid level between
material shall conform to the properties given in Table 1.
the 0 and 5 mL marks of a 10 mL measuring pipet. Calculate
4.3.2 If the mixture is to be used in its dough stage, the
the percent change as follows:
material shall conform to the properties given in Table 1.
t 2 t
a b
% Change 5 3 100 (1)
4.3.3 If the mixture can be used in either its predough or
t
b
dough stages, separate units must be tested for compliance with
4.3.1 and 4.3.2. where:
t = flow time before heating, and
4.4 Cured Polymer— The material after setting shall con-
b
t = flow time after heating exposure (4.1.2) of 60 6 2°C
form to the properties given in Table 2.
a
for 48 h in the dark in a closed container.
5. Weights and Permissible Variations
7.3.1 An alternative method for viscosity may be used if it
5.1 Weight and volume measurements shall be made on the can be demonstrated to yield similar results. Both shall comply
respective powder and liquid components of five units (see
to the less than 10 % change specified (4.1.2).
3.1). These units may be subsequently utilized in any of the 7.4 The components of the two units shall be tested for
nonsterile tests of this specification.
sterility in accordance with the test methods described in U.S.
5.2 The weights, or volume of the powder and liquid Pharmacopoeia,“ Sterility Tests” (3).
components, or both, shall not deviate by more than 5 % from 7.5 Doughing Time:
those stated on the package (9.2.2), of each of five units. 7.5.1 Environment— All equipment, mixing surfaces, and
5.3 Where a radiopaque material is supplied for addition to material (unit size) shall be maintained at 23 6 1°C at least 2
the powder at the discretion of the surgeon, the weight or
h prior to testing and tests shall be conducted at 23 6 1°C. The
volume percent of the radiopaque material shall not deviate by relative humidity shall be 50 6 10 %.
more than 15 % from the value stated on the package (9.2.3).
7.5.2 Mix all the powder and liquid of a single unit together
as directed by the manufacturer’s instructions (see 8.2). Start a
6. Sampling
stop watch at the onset of combining the liquid to the powder
6.1 Units of powder and liquid shall be procured to provide
and read all subsequent times from this stop watch. Approxi-
sufficient material for all the tests of this specification. The
mately 1.5 min after the onset of mixing, gently probe the
units shall be obtained from regular retail distribution channels.
mixture with a non-powdered surgically gloved (latex) finger.
Provided no repeat tests are required, this will amount to
Take visual notice as to the formation of fibers between the
between seven and ten units.
surface of the mix and the finger as it leaves the surface. Repeat
6.2 It will only be necessary to maintain sterility in tests
this process from that time on at 15 s intervals with a clean
described in 7.4. All other tests described in this specification
portion of the glove until the gloved finger separates cleanly.
need not be conducted under sterile conditions.
Denote the time at which this is first observed as the doughing
time. Mix the mixture between determinations to expose fresh
7. Test Methods and Sample Size
material for each probing.
7.1 Maintain all equipment, mixing surfaces, and materials
7.5.3 Determine the average doughing time from two sepa-
at 23 6 2°C at least 2 h prior to testing and conduct all tests at
rate units.
23 6 2°C and 50 6 10 % relative humidity unless otherwise
7.5.4 The two values found shall agree within 30 s of each
specified.
other, otherwise repeat the test on two additional units. Report
the average of all four tests and the range of values.
TABLE 1 Requirements for Powder Liquid Mixture
7.5.5 Report the doughing time to the nearest 15 s as the
Dough Usage,
Extrusion,
average of all determinations. Maximum and minimum values
Property Intrusion
Viscosity Tests
Tests
of doughing times measured shall not differ by more than 61 ⁄2
Max Dough Time, min. 5.0 5.0 min from the average.
Setting Time Range, min. 5 to 15 5 to 15
7.6 Exothermic Temperature—Within 1 min after doughing
Temperature, max., °C 90 90
time, gently pack approximately 25 g of the dough described in
Intrusion, min., mm . 2.0
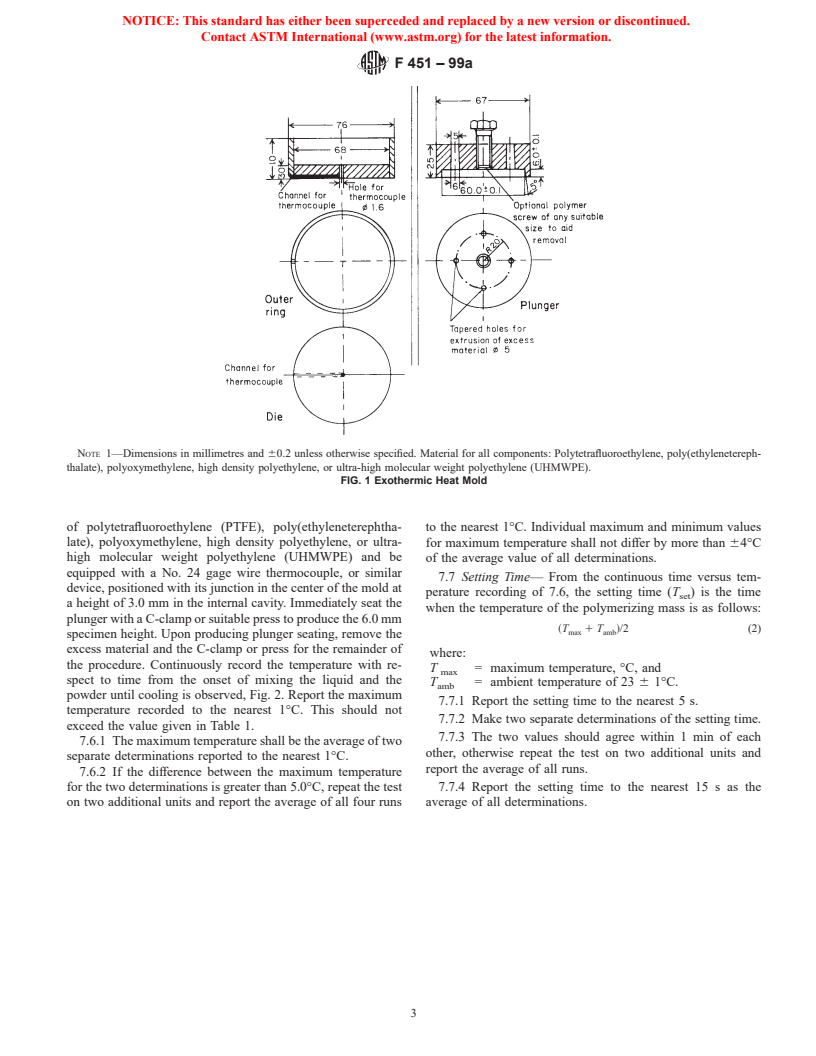
7.5 into the mold described in Fig. 1. This mold shall be made
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F 451 – 99a
NOTE 1—Dimensions in millimetres and 60.2 unless otherwise specified. Material for all components: Polytetrafluoroethylene, poly(ethylenetereph-
thalate), polyoxymethylene, high density polyethylene, or ultra-high molecular weight polyethylene (UHMWPE).
FIG. 1 Exothermic Heat Mold
of polytetrafluoroethylene (PTFE), poly(ethyleneterephtha- to the nearest 1°C. Individual maximum and minimum values
late), polyoxymethylene, high density polyethylene, or ultra- for maximum temperature shall not differ by more than 64°C
high molecular weight polyethylene (UHMWPE) and be of the average value of all determinations.
equipped with a No. 24 gage wire thermocouple, or similar
7.7 Setting Time— From the continuous time versus tem-
device, positioned with its junction in the center of the mold at
perature recording of 7.6, the setting time (T ) is the time
set
a height of 3.0 mm in the internal cavity. Immediately seat the
when the temperature of the polymerizing mass is as follows:
plunger with a C-clamp or suitable press to produce the 6.0 mm
~T 1 T !/2 (2)
max amb
specimen height. Upon producing plunger seating, remove the
excess material and the C-clamp or press for the remainder of
where:
the procedure. Continuously record the temperature with re-
T = maximum temperature, °C, and
max
spect to time from the onset of mixing the liquid and the
T = ambient temperature of 23 6 1°C.
amb
powder until cooling is observed, Fig. 2. Report the maximum
7.7.1 Report the setting time to the nearest 5 s.
temperature recorded to the nearest 1°C. This should not
7.7.2 Make two separate determinations of the setting time.
exceed the value given in Table 1.
7.7.3 The two values should agree within 1 min of each
7.6.1 The maximum temperature shall be the average of two
other, otherwise repeat the test on two additional units and
separate determinations reported to the nearest 1°C.
report the average of all runs.
7.6.2 If the difference between the maximum temperature
for the two determinations is greater than 5.0°C, repeat the test 7.7.4 Report the setting time to the nearest 15 s as the
on two additional units and report the average of all four runs average of all determinations.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F 451 – 99a
FIG. 2 Continuous Temperature Record
7.8 Flow Properties and Viscosity Determination—The kinetic energy losses, may be calculated. In such cases, the
manufacturer must specify whether the cement may be used in exact details of the mode of correction must be reported. Some
its pre-dough or dough state, or both. The determination of its correction factors which may apply are:
usage dictates which of the following tests the cement should
((a) (a) Piston friction,
comply with. If the mixture is to be utilized in the pre-dough
((b) (b) Plunger back flow,
stage, use the extrusion, viscosity test (7.8.1) and Table 1. If the
((c) (c) Cement compressibility,
mixture is to be utilized in the dough stage, use the intrusion
((d) (d) Barrel back pressure,
test (7.8.2) and Table 1. If the mixture is to be used as a dual
((e) (e) Capillary entrance effects (Bagley correction) (6),
usage cement, then both the extrusion (7.8.1) and intrusion
((f) (f) Rabinowitsch shear rate correction (7).
(7.8.2) tests must be performed.
7.8.2 Procedure:
7.8.1 Extrusion, Viscosity:
7.8.1.1 Apparatus: 7.8.2.1 Select conditions of temperature and shear stress or
shear rate in accordance with expected usage so that the flow
7.8.1.1.1 Rheometer—Any capillary rheometer is satisfac-
tory in which acrylic bone cement can be forced from a rate will fall within desired limits.
reservoir through a capillary die and in which temperature,
7.8.2.2 Inspect the rheometer and clean it if necessary.
applied force, output rate, and barrel and die dimensions can be
Ensure that previous cleaning procedures and usage have not
controlled and measured accurately. Equipment that provides a
changed the dimensions or caused scratches or defects in the
constant shear rate has been shown to be equally useful. The
capillary or apparatus. Make the necessary measurements on
capillary die of the rheometer shall have a smooth straight bore
the apparatus for future calculati
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.