ASTM G102-89(2004)e1
(Practice)Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements
Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements
SIGNIFICANCE AND USE
Electrochemical corrosion rate measurements often provide results in terms of electrical current. Although the conversion of these current values into mass loss rates or penetration rates is based on Faraday’Law, the calculations can be complicated for alloys and metals with elements having multiple valence values. This practice is intended to provide guidance in calculating mass loss and penetration rates for such alloys. Some typical values of equivalent weights for a variety of metals and alloys are provided.
Electrochemical corrosion rate measurements may provide results in terms of electrical resistance. The conversion of these results to either mass loss or penetration rates requires additional electrochemical information. Some approaches for estimating this information are given.
Use of this practice will aid in producing more consistent corrosion rate data from electrochemical results. This will make results from different studies more comparable and minimize calculation errors that may occur in transforming electrochemical results to corrosion rate values.
SCOPE
1.1 This practice covers the providing of guidance in converting the results of electrochemical measurements to rates of uniform corrosion. Calculation methods for converting corrosion current density values to either mass loss rates or average penetration rates are given for most engineering alloys. In addition, some guidelines for converting polarization resistance values to corrosion rates are provided.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information.
´1
Designation:G102–89(Reapproved2004)
Standard Practice for
Calculation of Corrosion Rates and Related Information
from Electrochemical Measurements
This standard is issued under the fixed designation G102; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
´ NOTE—Information updated editorially in November 2004.
1. Scope alloys. Some typical values of equivalent weights for a variety
of metals and alloys are provided.
1.1 This practice covers the providing of guidance in
3.2 Electrochemical corrosion rate measurements may pro-
convertingtheresultsofelectrochemicalmeasurementstorates
vide results in terms of electrical resistance.The conversion of
of uniform corrosion. Calculation methods for converting
these results to either mass loss or penetration rates requires
corrosion current density values to either mass loss rates or
additional electrochemical information. Some approaches for
averagepenetrationratesaregivenformostengineeringalloys.
estimating this information are given.
In addition, some guidelines for converting polarization resis-
3.3 Use of this practice will aid in producing more consis-
tance values to corrosion rates are provided.
tent corrosion rate data from electrochemical results. This will
2. Referenced Documents make results from different studies more comparable and
minimize calculation errors that may occur in transforming
2.1 ASTM Standards:
electrochemical results to corrosion rate values.
D2776 Test Methods for Corrosivity of Water in the Ab-
sence of Heat Transfer (Electrical Methods)
4. Corrosion Current Density
G1 Practice for Preparing, Cleaning, and Evaluating Corro-
4.1 Corrosioncurrentvaluesmaybeobtainedfromgalvanic
sion Test Specimens
cells and polarization measurements, including Tafel extrapo-
G5 Reference Test Method for Making Potentiostatic and
lations or polarization resistance measurements. (See Refer-
Potentiodynamic Anodic Polarization Measurements
enceTestMethodG5andPracticeG59forexamples.)Thefirst
G59 Test Method for Conducting Potentiodynamic Polar-
step is to convert the measured or estimated current value to
ization Resistance Measurements
current density. This is accomplished by dividing the total
3. Significance and Use
current by the geometric area of the electrode exposed to the
solution. The surface roughness is generally not taken into
3.1 Electrochemicalcorrosionratemeasurementsoftenpro-
accountwhencalculatingthecurrentdensity.Itisassumedthat
vide results in terms of electrical current. Although the con-
the current distributes uniformly across the area used in this
version of these current values into mass loss rates or penetra-
calculation.Inthecaseofgalvaniccouples,theexposedareaof
tion rates is based on Faraday’s Law, the calculations can be
the anodic specimen should be used. This calculation may be
complicated for alloys and metals with elements having
expressed as follows:
multiple valence values. This practice is intended to provide
guidanceincalculatingmasslossandpenetrationratesforsuch I
cor
i 5 (1)
cor
A
This practice is under the jurisdiction ofASTM Committee G01 on Corrosion
where:
ofMetalsandisthedirectresponsibilityofSubcommitteeG01.11onElectrochemi-
i = corrosion current density, µA/cm ,
cor
cal Measurements in Corrosion Testing.
I = total anodic current, µA, and
cor
Current edition approved Nov 1, 2004. Published November 2004. Originally
A = exposed specimen area, cm .
approved in 1989. Last previous edition approved in 1999 as G102– 89 (1999).
DOI: 10.1520/G0102-89R04E01. Other units may be used in this calculation. In some
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
computerized polarization equipment, this calculation is made
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
automatically after the specimen area is programmed into the
Standards volume information, refer to the standard’s Document Summary page on
computer. A sample calculation is given in Appendix X1.
the ASTM website.
Withdrawn.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
´1
G102–89 (2004)
4.2 Equivalent Weight—Equivalent weight, EW, may be
W = the atomic weight of the element, and
thought of as the mass of metal in grams that will be oxidized
n = thenumberofelectronsrequiredtooxidizeanatomof
by the passage of one Faraday (96 489 6 2 C (amp-sec)) of
the element in the corrosion process, that is, the
electric charge.
valence of the element.
4.3 For alloys, the equivalent weight is more complex. It is
NOTE 1—The value of EW is not dependent on the unit system chosen
usually assumed that the process of oxidation is uniform and
and so may be considered dimensionless.
doesnotoccurselectivelytoanycomponentofthealloy.Ifthis
For pure elements, the equivalent weight is given by:
is not true, then the calculation approach will need to be
W
adjusted to reflect the observed mechanism. In addition, some
EW 5 (2)
n
rationale must be adopted for assigning values of n to the
elementsinthealloybecausemanyelementsexhibitmorethan
where:
one valence value.
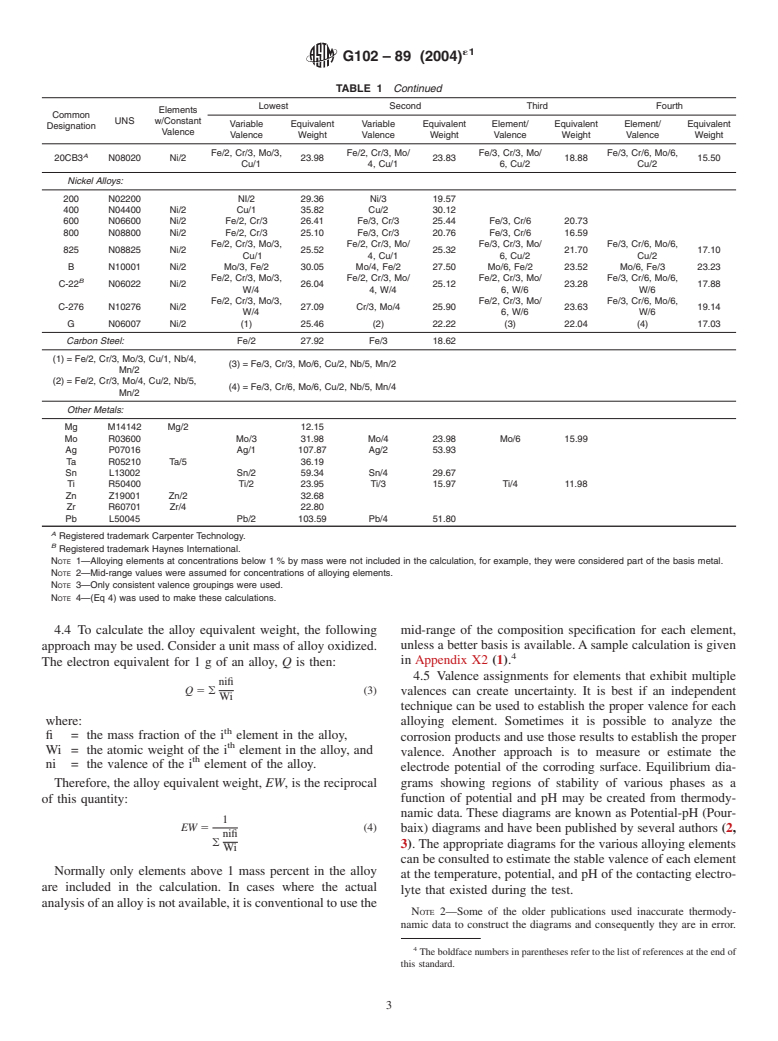
TABLE 1 Equivalent Weight Values for a Variety of Metals and Alloys
Lowest Second Third Fourth
Elements
Common
UNS w/Constant
Variable Equivalent Variable Equivalent Element/ Equivalent Element/ Equivalent
Designation
Valence
Valence Weight Valence Weight Valence Weight Valence Weight
Aluminum Alloys:
A
AA1100 A91100 Al/3 8.99
AA2024 A92024 Al/3, Mg/2 Cu/1 9.38 Cu/2 9.32
AA2219 A92219 Al/3 Cu/1 9.51 Cu/2 9.42
AA3003 A93003 Al/3 Mn/2 9.07 Mn/4 9.03 Mn 7 8.98
AA3004 A93004 Al/3, Mg/2 Mn/2 9.09 Mn/4 9.06 Mn 7 9.00
AA5005 A95005 Al/3, Mg/2 9.01
AA5050 A95050 Al/3, Mg/2 9.03
AA5052 A95052 Al/3, Mg/2 9.05
AA5083 A95083 Al/3, Mg/2 9.09
AA5086 A95086 Al/3, Mg/2 9.09
AA5154 A95154 Al/3, Mg/2 9.08
AA5454 A95454 Al/3, Mg/2 9.06
AA5456 A95456 Al/3, Mg/2 9.11
AA6061 A96061 Al/3, Mg/2 9.01
Al/3, Mg/2,
AA6070 A96070 8.98
Si/4
AA6101 A96161 Al/3 8.99
AA7072 A97072 Al/3, Zn/2 9.06
Al/3, Zn/2,
AA7075 A97075 Cu/1 9.58 Cu/2 9.55
Mg/2
Al/3, Zn/2,
AA7079 A97079 9.37
Mg/2
Al/3, Zn/2,
AA7178 A97178 Cu/1 9.71 Cu/2 9.68
Mg/2
Copper Alloys:
CDA110 C11000 Cu/1 63.55 Cu/2 31.77
CDA220 C22000 Zn/2 Cu/1 58.07 Cu/2 31.86
CDA230 C23000 Zn/2 Cu/1 55.65 Cu/2 31.91
CDA260 C26000 Zn/2 Cu/1 49.51 Cu/2 32.04
CDA280 C28000 Zn/2 Cu/1 46.44 Cu/2 32.11
CDA444 C44300 Zn/2 Cu/1, Sn/2 50.42 Cu/1, Sn/4 50.00 Cu/2, Sn/4 32.00
CDA687 C68700 Zn/2, Al/3 Cu/1 48.03 Cu/2 30.29
CDA608 C60800 Al/3 Cu/1 47.114 Cu/2 27.76
CDA510 C51000 Cu/1, Sn/2 63.32 Cu/1, Sn/4 60.11 Cu/2, Sn/4 31.66
CDA524 C52400 Cu/1, Sn/2 63.10 Cu/1, Sn/4 57.04 Cu/2, Sn/4 31.55
CDA655 C65500 Si/4 Cu/1 50.21 Cu/2 28.51
CDA706 C70600 Ni/2 Cu/1 56.92 Cu/2 31.51
CDA715 C71500 Ni/2 Cu/1 46.69 Cu/2 30.98
CDA752 C75200 Ni/2, Zn/2 Cu/1 46.38 Cu/2 31.46
Stainless Steels:
304 S30400 Ni/2 Fe/2, Cr/3 25.12 Fe/3, Cr/3 18.99 Fe/3, Cr/6 15.72
321 S32100 Ni/2 Fe/2, Cr/3 25.13 Fe/3, Cr/3 19.08 Fe/3, Cr/6 15.78
309 S30900 Ni/2 Fe/2, Cr/3 24.62 Fe/3, Cr/3 19.24 Fe/3, Cr/6 15.33
310 S31000 Ni/2 Fe/2, Cr/3 24.44 Fe/3, Cr/3 19.73 Fe/3, Cr/6 15.36
316 S31600 Ni/2 Fe/2, Cr/3, Mo/3 25.50 Fe/2, Cr/3, Mo/4 25.33 Fe/3, Cr/6, Mo/6 19.14 Fe/3, Cr/6, Mo/6 16.111
317 S31700 Ni/2 Fe/2, Cr/3, Mo/3 25.26 Fe/2, Cr/3, Mo/4 25.03 Fe/3, Cr/3, Mo/6 19.15 Fe/3, Cr/6, Mo/6 15.82
410 S41000 Fe/2, Cr/3 25.94 Fe/3, Cr/3 18.45 Fe/3, Cr/6 16.28
430 S43000 Fe/2, Cr/3 25.30 Fe/3, Cr/3 18.38 Fe/3, Cr/6 15.58
446 S44600 Fe/2, Cr/3 24.22 Fe/3, Cr/3 18.28 Fe/3, Cr/6 14.46
´1
G102–89 (2004)
TABLE 1 Continued
Lowest Second Third Fourth
Elements
Common
UNS w/Constant
Variable Equivalent Variable Equivalent Element/ Equivalent Element/ Equivalent
Designation
Valence
Valence Weight Valence Weight Valence Weight Valence Weight
Fe/2, Cr/3, Mo/3, Fe/2, Cr/3, Mo/ Fe/3, Cr/3, Mo/ Fe/3, Cr/6, Mo/6,
A
20CB3 N08020 Ni/2 23.98 23.83 18.88 15.50
Cu/1 4, Cu/1 6, Cu/2 Cu/2
Nickel Alloys:
200 N02200 NI/2 29.36 Ni/3 19.57
400 N04400 Ni/2 Cu/1 35.82 Cu/2 30.12
600 N06600 Ni/2 Fe/2, Cr/3 26.41 Fe/3, Cr/3 25.44 Fe/3, Cr/6 20.73
800 N08800 Ni/2 Fe/2, Cr/3 25.10 Fe/3, Cr/3 20.76 Fe/3, Cr/6 16.59
Fe/2, Cr/3, Mo/3, Fe/2, Cr/3, Mo/ Fe/3, Cr/3, Mo/ Fe/3, Cr/6, Mo/6,
825 N08825 Ni/2 25.52 25.32 21.70 17.10
Cu/1 4, Cu/1 6, Cu/2 Cu/2
B N10001 Ni/2 Mo/3, Fe/2 30.05 Mo/4, Fe/2 27.50 Mo/6, Fe/2 23.52 Mo/6, Fe/3 23.23
Fe/2, Cr/3, Mo/3, Fe/2, Cr/3, Mo/ Fe/2, Cr/3, Mo/ Fe/3, Cr/6, Mo/6,
B
C-22 N06022 Ni/2 26.04 25.12 23.28 17.88
W/4 4, W/4 6, W/6 W/6
Fe/2, Cr/3, Mo/3, Fe/2, Cr/3, Mo/ Fe/3, Cr/6, Mo/6,
C-276 N10276 Ni/2 27.09 Cr/3, Mo/4 25.90 23.63 19.14
W/4 6, W/6 W/6
G N06007 Ni/2 (1) 25.46 (2) 22.22 (3) 22.04 (4) 17.03
Carbon Steel: Fe/2 27.92 Fe/3 18.62
(1) = Fe/2, Cr/3, Mo/3, Cu/1, Nb/4,
(3) = Fe/3, Cr/3, Mo/6, Cu/2, Nb/5, Mn/2
Mn/2
(2) = Fe/2, Cr/3, Mo/4, Cu/2, Nb/5,
(4) = Fe/3, Cr/6, Mo/6, Cu/2, Nb/5, Mn/4
Mn/2
Other Metals:
Mg M14142 Mg/2 12.15
Mo R03600 Mo/3 31.98 Mo/4 23.98 Mo/6 15.99
Ag P07016 Ag/1 107.87 Ag/2 53.93
Ta R05210 Ta/5 36.19
Sn L13002 Sn/2 59.34 Sn/4 29.67
Ti R50400 Ti/2 23.95 Ti/3 15.97 Ti/4 11.98
Zn Z19001 Zn/2 32.68
Zr R60701 Zr/4 22.80
Pb L50045 Pb/2 103.59 Pb/4 51.80
A
Registered trademark Carpenter Technology.
B
Registered trademark Haynes International.
NOTE 1—Alloying elements at concentrations below 1 % by mass were not included in the calculation, for example, they were considered part of the basis metal.
NOTE 2—Mid-range values were assumed for concentrations of alloying elements.
NOTE 3—Only consistent valence groupings were used.
NOTE 4—(Eq 4) was used to make these calculations.
4.4 To calculate the alloy equivalent weight, the following mid-range of the composition specification for each element,
approach may be used. Consider a unit mass of alloy oxidized. unless a better basis is available.Asample calculation is given
in Appendix X2 (1).
The electron equivalent for1gofan alloy, Q is then:
4.5 Valence assignments for elements that exhibit multiple
nifi
Q 5 ( (3) valences can create uncertainty. It is best if an independent
Wi
technique can be used to establish the proper valence for each
where: alloying element. Sometimes it is possible to analyze the
th
fi = the mass fraction of the i element in the alloy,
corrosion products and use those results to establish the proper
th
Wi = the atomic weight of the i element in the alloy, and
valence. Another approach is to measure or estimate the
th
ni = the valence of the i element of the alloy.
electrode potential of the corroding surface. Equilibrium dia-
Therefore, the alloy equivalent weight, EW, is the reciprocal grams showing regions of stability of various phases as a
of this quantity: function of potential and pH may be created from thermody-
namic data. These diagrams are known as Potential-pH (Pour-
EW 5 (4) baix) diagrams and have been published by several authors (2,
nifi
(
3).The appropriate diagrams for the various alloying elements
Wi
canbeconsultedtoestimatethestablevalenceofeachelement
Normally only elements above 1 mass percent in the alloy
at the temperature, potential, and pH of the contacting electro-
are included in the calculation. In cases where the actual
lyte that existed during the test.
analysisofanalloyisnotavailable,itisconventionaltousethe
NOTE 2—Some of the older publications used inaccurate thermody-
namic data to construct the diagrams and consequently they are in error.
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
this standard.
´1
G102–89 (2004)
4.6 Some typical values of EW for a variety of metals and izationusingasinglesmallpotentialstep, DE,usuallyeither10
alloys are given in Table 1. mV or−10 mV, (see Test Method D2776). Values of 65 and
4.7 Calculation of Corrosion Rate—Faraday’s Law can be 620 mV are also commonly used. In this case, the specimen
used to calculate the corrosion rate, either in terms of penetra- current, DI, is measured after steady state occurs, and DE/DI is
tion rate (CR) or mass loss rate (MR) (4): calculated. Potentiodynamic measurements yield curves of I
versus E and the reciprocal of the slope of the curve (dE/dI) at
i
cor
CR 5K EW (5)
1 the corrosion potential is measured. In most programmable
r
potentiodynamic polarization equipment, the current is con-
MR 5K i EW (6)
2 cor
verted to current density automatically and the resulting plot is
where:
of i versus E. In this case, the polarization resistance is given
CR is given in mm/yr, i in µA/cm ,
cor
by dE/di at the corrosion potential and 5.2 is not applicable.
5.2 It is necessary to multiply the dE/dI or DE/DI value
−3 calculated above by the exposed specimen geometric area to
K = 3.27 310 , mm g/µA cm yr (Note 3),
obtain the polarization resistance. This is equivalent to the
r = density in g/cm , (see Practice G1 for density values
calculation shown in 4.1 for current density.
for many metals and alloys used in corrosion test-
5.3 The Stern-Geary constant B must be estimated or
ing),
calculatedtoconvertpolarizationresistancevaluestocorrosion
MR = g/m d, and
−3 2 2
current density (6, 8).
K = 8.954 310 ,gcm /µA md(Note 3).
5.3.1 Calculate Stern-Geary constants from known Tafel
NOTE 3—EW is considered dimensionless in these calculations.
slopes where both cathodic and anodic reactions are activation
Other values for K and K for different unit systems are
controlled, that is, there are distinct linear regions near the
1 2
given in Table 2.
corrosion potential on an E log i plot:
4.8 Errors that may arise from this procedure are discussed
babc
B 5 (7)
below.
2.303 ~ba 1bc!
4.8.1 Assignment of incorrect valence values may cause
serious errors (5). where:
ba = slope of the anodic Tafel reaction, when plotted on
4.8.2 The calculation of penetration or mass loss from
base 10 logarithmic paper in V/decade,
electrochemical measurements, as described in this standard,
bc = slope of the cathodic Tafel reaction when plotted on
assumes that uniform corrosion is occurring. In cases where
base 10 logarithmic paper in V/decade, and
non-uniformcorrosionprocessesareoccurring,theuseofthese
B = Stern-Geary constant, V.
methodsmayresultinasubstantialunderestimationofthetrue
5.3.2 In cases where one of the reactions is purely diffusion
values.
controlled, the Stern-Geary constant may be calculated:
4.8.3 Alloys that include large quantities of metalloids or
oxidized materials may not be able to be treated by the above
b
B 5 (8)
2.303
procedure.
4.8.4 Corrosionratescalculatedbythemetho
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.