ASTM G102-89(2010)
(Practice)Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements
Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements
SIGNIFICANCE AND USE
Electrochemical corrosion rate measurements often provide results in terms of electrical current. Although the conversion of these current values into mass loss rates or penetration rates is based on Faraday's Law, the calculations can be complicated for alloys and metals with elements having multiple valence values. This practice is intended to provide guidance in calculating mass loss and penetration rates for such alloys. Some typical values of equivalent weights for a variety of metals and alloys are provided.
Electrochemical corrosion rate measurements may provide results in terms of electrical resistance. The conversion of these results to either mass loss or penetration rates requires additional electrochemical information. Some approaches for estimating this information are given.
Use of this practice will aid in producing more consistent corrosion rate data from electrochemical results. This will make results from different studies more comparable and minimize calculation errors that may occur in transforming electrochemical results to corrosion rate values.
SCOPE
1.1 This practice covers the providing of guidance in converting the results of electrochemical measurements to rates of uniform corrosion. Calculation methods for converting corrosion current density values to either mass loss rates or average penetration rates are given for most engineering alloys. In addition, some guidelines for converting polarization resistance values to corrosion rates are provided.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: G102 − 89(Reapproved 2010)
Standard Practice for
Calculation of Corrosion Rates and Related Information
from Electrochemical Measurements
This standard is issued under the fixed designation G102; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope multiple valence values. This practice is intended to provide
guidanceincalculatingmasslossandpenetrationratesforsuch
1.1 This practice covers the providing of guidance in
alloys. Some typical values of equivalent weights for a variety
convertingtheresultsofelectrochemicalmeasurementstorates
of metals and alloys are provided.
of uniform corrosion. Calculation methods for converting
corrosion current density values to either mass loss rates or
3.2 Electrochemical corrosion rate measurements may pro-
averagepenetrationratesaregivenformostengineeringalloys.
vide results in terms of electrical resistance.The conversion of
In addition, some guidelines for converting polarization resis-
these results to either mass loss or penetration rates requires
tance values to corrosion rates are provided.
additional electrochemical information. Some approaches for
estimating this information are given.
1.2 The values stated in SI units are to be regarded as
standard. No other units of measurement are included in this
3.3 Use of this practice will aid in producing more consis-
standard.
tent corrosion rate data from electrochemical results. This will
make results from different studies more comparable and
2. Referenced Documents
minimize calculation errors that may occur in transforming
2.1 ASTM Standards:
electrochemical results to corrosion rate values.
D2776Methods of Test for Corrosivity of Water in the
Absence of Heat Transfer (Electrical Methods) (With-
4. Corrosion Current Density
drawn 1991)
4.1 Corrosioncurrentvaluesmaybeobtainedfromgalvanic
G1Practice for Preparing, Cleaning, and Evaluating Corro-
cells and polarization measurements, including Tafel extrapo-
sion Test Specimens
lations or polarization resistance measurements. (See Refer-
G5Reference Test Method for Making Potentiodynamic
enceTestMethodG5andPracticeG59forexamples.)Thefirst
Anodic Polarization Measurements
step is to convert the measured or estimated current value to
G59TestMethodforConductingPotentiodynamicPolariza-
current density. This is accomplished by dividing the total
tion Resistance Measurements
current by the geometric area of the electrode exposed to the
3. Significance and Use
solution. The surface roughness is generally not taken into
accountwhencalculatingthecurrentdensity.Itisassumedthat
3.1 Electrochemicalcorrosionratemeasurementsoftenpro-
the current distributes uniformly across the area used in this
vide results in terms of electrical current. Although the con-
calculation.Inthecaseofgalvaniccouples,theexposedareaof
version of these current values into mass loss rates or penetra-
the anodic specimen should be used. This calculation may be
tion rates is based on Faraday’s Law, the calculations can be
expressed as follows:
complicated for alloys and metals with elements having
I
cor
i 5 (1)
cor
A
This practice is under the jurisdiction ofASTM Committee G01 on Corrosion
of Metalsand is the direct responsibility of Subcommittee G01.11 on Electrochemi-
where:
cal Measurements in Corrosion Testing.
I = corrosion current density, µA/cm ,
Current edition approved May 1, 2010. Published May 2010. Originally
cor
ε1
approved in 1989. Last previous edition approved in 2004 as G102–89(2004) .
I = total anodic current, µA, and
cor
DOI: 10.1520/G0102-89R10.
A = exposed specimen area, cm .
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Other units may be used in this calculation. In some
Standards volume information, refer to the standard’s Document Summary page on
computerized polarization equipment, this calculation is made
the ASTM website.
3 automatically after the specimen area is programmed into the
The last approved version of this historical standard is referenced on
www.astm.org. computer. A sample calculation is given in Appendix X1.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G102 − 89 (2010)
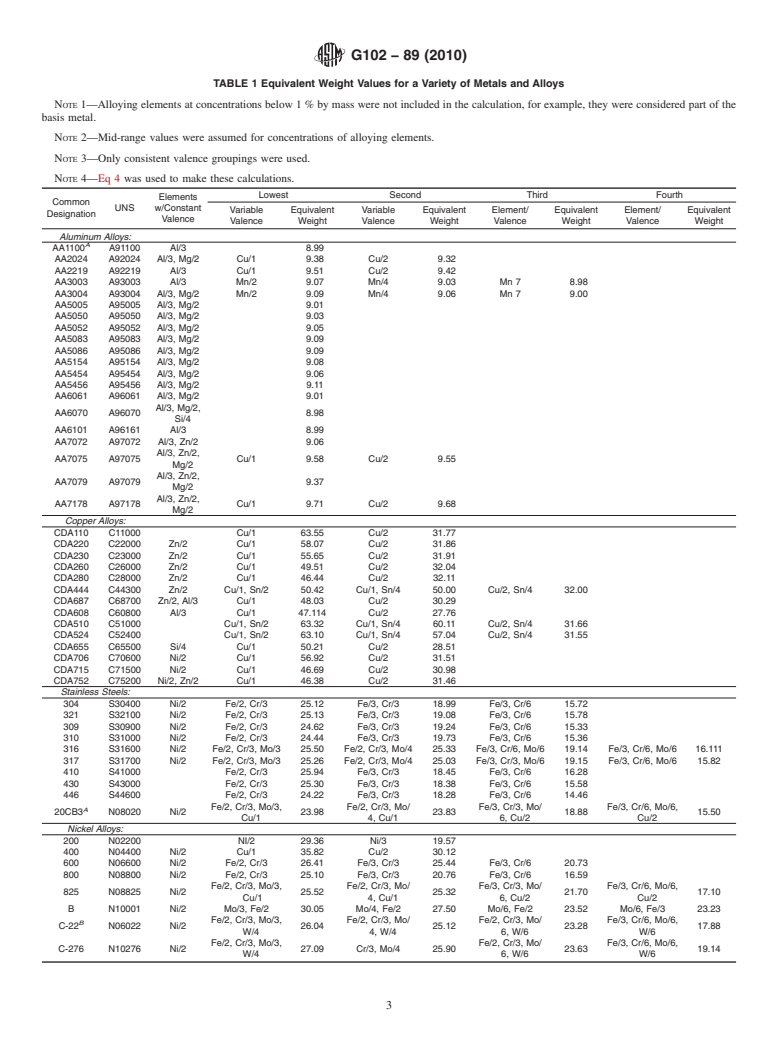
4.2 Equivalent Weight—Equivalent weight, EW, may be namic data. These diagrams are known as Potential-pH (Pour-
thought of as the mass of metal in grams that will be oxidized baix) diagrams and have been published by several authors (2,
by the passage of one Faraday (96 489 6 2 C (amp-sec)) of 3).The appropriate diagrams for the various alloying elements
electric charge. canbeconsultedtoestimatethestablevalenceofeachelement
at the temperature, potential, and pH of the contacting electro-
NOTE 1—The value of EW is not dependent on the unit system chosen
lyte that existed during the test.
and so may be considered dimensionless.
For pure elements, the equivalent weight is given by: NOTE 2—Some of the older publications used inaccurate thermody-
namic data to construct the diagrams and consequently they are in error.
W
EW 5 (2)
4.6 Some typical values of EW for a variety of metals and
n
alloys are given in Table 1.
where:
4.7 Calculation of Corrosion Rate—Faraday’s Law can be
W = the atomic weight of the element, and
used to calculate the corrosion rate, either in terms of penetra-
n = the number of electrons required to oxidize an atom of
tion rate (CR) or mass loss rate (MR) (4):
theelementinthecorrosionprocess,thatis,thevalence
i
of the element. cor
CR 5 K EW (5)
ρ
4.3 For alloys, the equivalent weight is more complex. It is
MR 5 K i EW (6)
usually assumed that the process of oxidation is uniform and 2 cor
doesnotoccurselectivelytoanycomponentofthealloy.Ifthis
where:
is not true, then the calculation approach will need to be
CR is given in mm/yr, i in µA/cm ,
cor
adjusted to reflect the observed mechanism. In addition, some
rationale must be adopted for assigning values of n to the
−3
K = 3.27×10 , mm g/µA cm yr (Note 3),
elementsinthealloybecausemanyelementsexhibitmorethan
ρ = density in g/cm , (see Practice G1 for density values
one valence value.
for many metals and alloys used in corrosion testing),
4.4 To calculate the alloy equivalent weight, the following
MR = g/m d, and
approach may be used. Consider a unit mass of alloy oxidized. −3 2 2
K = 8.954×10 ,gcm /µA md(Note 3).
The electron equivalent for1gofan alloy, Q is then:
NOTE 3—EW is considered dimensionless in these calculations.
nifi
Other values for K and K for different unit systems are
1 2
Q 5 (3)
(
Wi
given in Table 2.
where:
4.8 Errors that may arise from this procedure are discussed
th
below.
fi = the mass fraction of the i element in the alloy,
th
4.8.1 Assignment of incorrect valence values may cause
Wi = the atomic weight of the i element in the alloy, and
th
ni = the valence of the i element of the alloy. serious errors (5).
4.8.2 The calculation of penetration or mass loss from
Therefore, the alloy equivalent weight, EW, is the reciprocal
electrochemical measurements, as described in this standard,
of this quantity:
assumes that uniform corrosion is occurring. In cases where
non-uniformcorrosionprocessesareoccurring,theuseofthese
EW 5 (4)
nifi
methodsmayresultinasubstantialunderestimationofthetrue
(
Wi
values.
4.8.3 Alloys that include large quantities of metalloids or
Normally only elements above 1 mass percent in the alloy
oxidized materials may not be able to be treated by the above
are included in the calculation. In cases where the actual
procedure.
analysisofanalloyisnotavailable,itisconventionaltousethe
4.8.4 Corrosionratescalculatedbythemethodabovewhere
mid-range of the composition specification for each element,
abrasionorerosionisasignificantcontributortothemetalloss
unless a better basis is available.Asample calculation is given
processmayyieldsignificantunderestimationofthemetalloss
in Appendix X2 (1).
rate.
4.5 Valence assignments for elements that exhibit multiple
valences can create uncertainty. It is best if an independent
5. Polarization Resistance
technique can be used to establish the proper valence for each
5.1 Polarization resistance values may be approximated
alloying element. Sometimes it is possible to analyze the
from either potentiodynamic measurements near the corrosion
corrosion products and use those results to establish the proper
potential (see Practice G59) or stepwise potentiostatic polar-
valence. Another approach is to measure or estimate the
ization using a single small potential step, ∆E, usually either
electrode potential of the corroding surface. Equilibrium dia-
10mVor−10mV,(seeTestMethodD2776).Valuesof 65and
grams showing regions of stability of various phases as a
620 mV are also commonly used. In this case, the specimen
function of potential and pH may be created from thermody-
current, ∆I, is measured after steady state occurs, and ∆E/∆I is
calculated. Potentiodynamic measurements yield curves of I
versus E and the reciprocal of the slope of the curve (dE/dI) at
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
this standard. the corrosion potential is measured. In most programmable
G102 − 89 (2010)
TABLE 1 Equivalent Weight Values for a Variety of Metals and Alloys
NOTE 1—Alloying elements at concentrations below 1% by mass were not included in the calculation, for example, they were considered part of the
basis metal.
NOTE 2—Mid-range values were assumed for concentrations of alloying elements.
NOTE 3—Only consistent valence groupings were used.
NOTE 4—Eq 4 was used to make these calculations.
Lowest Second Third Fourth
Elements
Common
UNS w/Constant
Variable Equivalent Variable Equivalent Element/ Equivalent Element/ Equivalent
Designation
Valence
Valence Weight Valence Weight Valence Weight Valence Weight
Aluminum Alloys:
A
AA1100 A91100 Al/3 8.99
AA2024 A92024 Al/3, Mg/2 Cu/1 9.38 Cu/2 9.32
AA2219 A92219 Al/3 Cu/1 9.51 Cu/2 9.42
AA3003 A93003 Al/3 Mn/2 9.07 Mn/4 9.03 Mn 7 8.98
AA3004 A93004 Al/3, Mg/2 Mn/2 9.09 Mn/4 9.06 Mn 7 9.00
AA5005 A95005 Al/3, Mg/2 9.01
AA5050 A95050 Al/3, Mg/2 9.03
AA5052 A95052 Al/3, Mg/2 9.05
AA5083 A95083 Al/3, Mg/2 9.09
AA5086 A95086 Al/3, Mg/2 9.09
AA5154 A95154 Al/3, Mg/2 9.08
AA5454 A95454 Al/3, Mg/2 9.06
AA5456 A95456 Al/3, Mg/2 9.11
AA6061 A96061 Al/3, Mg/2 9.01
Al/3, Mg/2,
AA6070 A96070 8.98
Si/4
AA6101 A96161 Al/3 8.99
AA7072 A97072 Al/3, Zn/2 9.06
Al/3, Zn/2,
AA7075 A97075 Cu/1 9.58 Cu/2 9.55
Mg/2
Al/3, Zn/2,
AA7079 A97079 9.37
Mg/2
Al/3, Zn/2,
AA7178 A97178 Cu/1 9.71 Cu/2 9.68
Mg/2
Copper Alloys:
CDA110 C11000 Cu/1 63.55 Cu/2 31.77
CDA220 C22000 Zn/2 Cu/1 58.07 Cu/2 31.86
CDA230 C23000 Zn/2 Cu/1 55.65 Cu/2 31.91
CDA260 C26000 Zn/2 Cu/1 49.51 Cu/2 32.04
CDA280 C28000 Zn/2 Cu/1 46.44 Cu/2 32.11
CDA444 C44300 Zn/2 Cu/1, Sn/2 50.42 Cu/1, Sn/4 50.00 Cu/2, Sn/4 32.00
CDA687 C68700 Zn/2, Al/3 Cu/1 48.03 Cu/2 30.29
CDA608 C60800 Al/3 Cu/1 47.114 Cu/2 27.76
CDA510 C51000 Cu/1, Sn/2 63.32 Cu/1, Sn/4 60.11 Cu/2, Sn/4 31.66
CDA524 C52400 Cu/1, Sn/2 63.10 Cu/1, Sn/4 57.04 Cu/2, Sn/4 31.55
CDA655 C65500 Si/4 Cu/1 50.21 Cu/2 28.51
CDA706 C70600 Ni/2 Cu/1 56.92 Cu/2 31.51
CDA715 C71500 Ni/2 Cu/1 46.69 Cu/2 30.98
CDA752 C75200 Ni/2, Zn/2 Cu/1 46.38 Cu/2 31.46
Stainless Steels:
304 S30400 Ni/2 Fe/2, Cr/3 25.12 Fe/3, Cr/3 18.99 Fe/3, Cr/6 15.72
321 S32100 Ni/2 Fe/2, Cr/3 25.13 Fe/3, Cr/3 19.08 Fe/3, Cr/6 15.78
309 S30900 Ni/2 Fe/2, Cr/3 24.62 Fe/3, Cr/3 19.24 Fe/3, Cr/6 15.33
310 S31000 Ni/2 Fe/2, Cr/3 24.44 Fe/3, Cr/3 19.73 Fe/3, Cr/6 15.36
316 S31600 Ni/2 Fe/2, Cr/3, Mo/3 25.50 Fe/2, Cr/3, Mo/4 25.33 Fe/3, Cr/6, Mo/6 19.14 Fe/3, Cr/6, Mo/6 16.111
317 S31700 Ni/2 Fe/2, Cr/3, Mo/3 25.26 Fe/2, Cr/3, Mo/4 25.03 Fe/3, Cr/3, Mo/6 19.15 Fe/3, Cr/6, Mo/6 15.82
410 S41000 Fe/2, Cr/3 25.94 Fe/3, Cr/3 18.45 Fe/3, Cr/6 16.28
430 S43000 Fe/2, Cr/3 25.30 Fe/3, Cr/3 18.38 Fe/3, Cr/6 15.58
446 S44600 Fe/2, Cr/3 24.22 Fe/3, Cr/3 18.28 Fe/3, Cr/6 14.46
Fe/2, Cr/3, Mo/3, Fe/2, Cr/3, Mo/ Fe/3, Cr/3, Mo/ Fe/3, Cr/6, Mo/6,
A
20CB3 N08020 Ni/2 23.98 23.83 18.88 15.50
Cu/1 4, Cu/1 6, Cu/2 Cu/2
Nickel Alloys:
200 N02200 NI/2 29.36 Ni/3 19.57
400 N04400 Ni/2 Cu/1 35.82 Cu/2 30.12
600 N06600 Ni/2 Fe/2, Cr/3 26.41 Fe/3, Cr/3 25.44 Fe/3, Cr/6 20.73
800 N08800 Ni/2 Fe/2, Cr/3 25.10 Fe/3, Cr/3 20.76 Fe/3, Cr/6 16.59
Fe/2, Cr/3, Mo/3, Fe/2, Cr/3, Mo/ Fe/3, Cr/3, Mo/ Fe/3, Cr/6, Mo/6,
825 N08825 Ni/2 25.52 25.32 21.70 17.10
Cu/1 4, Cu/1 6, Cu/2 Cu/2
B N10001 Ni/2 Mo/3, Fe/2 30.05 Mo/4, Fe/2 27.50 Mo/6, Fe/2 23.52 Mo/6, Fe/3 23.23
Fe/2, Cr/3, Mo/3, Fe/2, Cr/3, Mo/ Fe/2, Cr/3, Mo/ Fe/3, Cr/6, Mo/6,
B
C-22 N06022 Ni/2 26.04 25.12 23.28 17.88
W/4 4, W/4 6, W/6 W/6
Fe/2, Cr/3, Mo/3, Fe/2, Cr/3, Mo/ Fe/3, Cr/6, Mo/6,
C-276 N10276 Ni/2 27.09 Cr/3, Mo/4 25.90 23.63 19.14
W/4 6, W/6 W/6
G102 − 89 (2010)
TABLE 1 Continued
Lowest Second Third Fourth
Elements
Common
UNS w/Constant
Variable Equivalent Variable Equivalent Element/ Equivalent Element/ Equivalent
Designation
Valence
Valence Weight Valence Weight Valence Weight Valence Weight
G N06007 Ni/2 (1) 25.46 (2) 22.22 (3) 22.04 (4) 17.03
Carbon Steel: Fe/2 27.92 Fe/3 18.62
(1) = Fe ⁄2, Cr/3, Mo/3, Cu/1, Nb/4,
(3) = Fe ⁄3, Cr/3, Mo/6, Cu/2, Nb/5, Mn/2
Mn/2
(2) = Fe ⁄2, Cr/3, Mo/4, Cu/2, Nb/5,
(4) = Fe ⁄3, Cr/6, Mo/6, Cu/2, Nb/5, Mn/4
Mn/2
Other Metals:
Mg M14142 Mg/2 12.15
Mo R03600 Mo/3 31.98 Mo/4 23.98 Mo/6 15.99
Ag P07016 Ag/1 107.87 Ag/2 53.93
Ta R05210 Ta/5 36.19
Sn L13002 Sn/2 59.34 Sn/4 29.67
Ti R50400 Ti/2 23.95 Ti/3 15.97 Ti/4 11.98
Zn Z19001 Zn/2 32.68
Zr R60701 Zr/4 22.80
Pb L50045 Pb/2 103.59 Pb/4 51.80
A
Registered trademark Carpenter Technology.
B
Registered trademark Haynes International.
TABLE 2 Values of Constants for Use in Faraday’s Equation Rate
5.3.2 In cases where one of the reactions is purely diffusion
A controlled, the Stern-Geary constant may be calculated:
Penetration
A
I Unit ρ Unit K Units of K
cor 1 1
b
Rate Unit (CR)
B 5 (8)
2 3
mpy µA/cm g/cm 0.1288 mpy g/µA cm
2.303
B 2B 3B
mm/yr A/m kg/m 327.2 mm kg/A m y
B 2 3 −3
mm/yr µA/cm g/cm 3.27 × 10 mm g/µA cm y
where:
B
b = the activation controlled Tafel slope in V/decade.
Mass Loss Rate
A
I Unit K Units of K
cor 2 2
Unit
5.3.3 It should be noted in this case that the corrosion
2 B 2B
g/m d A/m 0.8953 g/Ad
current density will be equal to the diffusion limited current
2 2 2 2
mg/dm d (mdd) µA/cm 0.0895 mg cm /µA dm d
2 2B −3 2 2
mg/dm d (mdd) A/m 8.953 × 10 mg m /A dm d densi
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.