ASTM D3326-07(2011)
(Practice)Standard Practice for Preparation of Samples for Identification of Waterborne Oils
Standard Practice for Preparation of Samples for Identification of Waterborne Oils
SIGNIFICANCE AND USE
Identification of a recovered oil is determined by comparison with known oils selected because of their possible relationship to the particular recovered oil, for example, suspected or questioned sources. Thus, samples of such known oils must be collected and submitted along with the unknown for analysis. It is unlikely that identification of the sources of an unknown oil by itself can be made without direct matching, that is, solely with a library of analyses.
SCOPE
1.1 This practice covers the preparation for analysis of waterborne oils recovered from water. The identification is based upon the comparison of physical and chemical characteristics of the waterborne oils with oils from suspect sources. These oils may be of petroleum or vegetable/animal origin, or both. Seven procedures are given as follows:
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D3326 −07 (Reapproved 2011)
Standard Practice for
Preparation of Samples for Identification of Waterborne
Oils
This standard is issued under the fixed designation D3326; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 This practice covers the preparation for analysis of
D95Test Method for Water in Petroleum Products and
waterborne oils recovered from water. The identification is
Bituminous Materials by Distillation
based upon the comparison of physical and chemical charac-
D1129Terminology Relating to Water
teristics of the waterborne oils with oils from suspect sources.
D1193Specification for Reagent Water
These oils may be of petroleum or vegetable/animal origin, or
D3325Practice for Preservation of Waterborne Oil Samples
both. Seven procedures are given as follows:
D3328Test Methods for Comparison of Waterborne Petro-
Sections
leum Oils by Gas Chromatography
Procedure A (for samples of more than 50-mL volume
containing significant quantities of hydrocarbons
D3414Test Method for Comparison of Waterborne Petro-
with boiling points above 280°C) 8 to 12
leum Oils by Infrared Spectroscopy
Procedure B (for samples containing significant quantities of
D3415Practice for Identification of Waterborne Oils
hydrocarbons with boiling points above 280°C) 13 to 17
Procedure C (for waterborne oils containing significant
D3650Test Method for Comparison of Waterborne Petro-
amounts of components boiling below 280°C and
leum Oils By Fluorescence Analysis
to mixtures of these and higher boiling components) 18 to 22
D4489Practices for Sampling of Waterborne Oils
Procedure D (for samples containing both petroleum and
vegetable/animal derived oils) 23 to 27
E1Specification for ASTM Liquid-in-Glass Thermometers
Procedure E (for samples of light crudes and medium distillate
E133Specification for Distillation Equipment
fuels) 28 to 34
Procedure F (for thin films of oil-on-water) 35 to 39
3. Terminology
Procedure G (for oil-soaked samples) 40 to 44
3.1 Definitions—For definitions of terms used in this
1.2 Procedures for the analytical examination of the water-
practice, refer to Terminology D1129.
borne oil samples are described in Practice D3415, D3328,
D3414, and D3650. Refer to the individual oil identification
3.2 Definitions of Terms Specific to This Standard:
test methods for the sample preparation method of choice.The
3.2.1 animal/vegetable-derived oils—a mixture made of
deasphalting effects of the sample preparation method should
mono-, di-, and triglyceride esters of fatty acids and other
be considered in selecting the best methods.
substances of animal or vegetable origin, or both.
1.3 The values stated in SI units are to be regarded as 3.2.2 Simulated weathering of waterborne oils by distilla-
standard. No other units of measurement are included in this tionconsidersonlytheeffectofevaporation,whichlikelyisthe
standard. most significant short-term weathering effect in the environ-
ment.
1.4 This standard does not purport to address all of the
3.2.3 Simulated weathering of waterborne oils by evapora-
safety concerns, if any, associated with its use. It is the
tion under ultraviolet light simulates the loss of light compo-
responsibility of the user of this standard to establish appro-
nents on weathering, as well as some oxidative weathering.
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use. Specific caution
4. Significance and Use
statements are given in Sections 6 and 32.
4.1 Identification of a recovered oil is determined by com-
parison with known oils selected because of their possible
relationship to the particular recovered oil, for example,
This practice is under the jurisdiction ofASTM Committee D19 on Water and
is the direct responsibility of Subcommittee D19.06 on Methods for Analysis for
Organic Substances in Water. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved May 1, 2011. Published June 2011. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 1974. Last previous edition approved in 2007 as D3326–07. DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/D3326-07R11. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3326−07 (2011)
NOTE 1—The boiling point may be ascertained by injecting the neat
suspectedorquestionedsources.Thus,samplesofsuchknown
samples into the gas chromatograph and checking the elution times above
oils must be collected and submitted along with the unknown
that of pentadecane on a nonpolar column.
for analysis. It is unlikely that identification of the sources of
8.2 Thepreparationofsamplescontainingmostlyhydrocar-
an unknown oil by itself can be made without direct matching,
that is, solely with a library of analyses. bons of boiling points below 280°C, such as petroleum
distillate fuels, is beyond the scope of this procedure (see
5. Reagents and Materials
Procedure C or E).
5.1 Purity of Reagents—Reagent grade chemicals shall be
9. Summary of Procedure
used in all tests. Unless otherwise indicated, it is intended that
all reagents shall conform to the specifications of the Commit-
9.1 A neat portion of the waterborne oil is retained. If not
teeonAnalyticalReagentsoftheAmericanChemicalSociety.
possible to obtain a neat portion, then retain a portion of the
Special ancillary procedures such as fluorescence may require
waterborne oil as received.This is to be used in those analyses
higher purity grades of solvents. Other grades may be used
performed on samples containing significant quantities of
provided it is first ascertained that the reagent is of sufficiently
hydrocarbons with boiling points below 280°C. Preparation of
high purity to permit its use without lessening the accuracy of
these samples is beyond the scope of this procedure, but are
the determination.
covered in Procedure C.
5.2 Purity of Water—Unless otherwise indicated, references
NOTE 2—Waterborne oil samples containing significant quantities of
to water shall be understood to mean reagent water that meets
hydrocarbons with boiling points below 280°C (see Note 1), such as
thepurityspecificationsofTypeIorTypeIIwater,asspecified
gasoline and kerosene, can usually be obtained as neat samples without
in Specification D1193. any sample preparation.
9.2 The waterborne oil sample is dissolved in an equal
6. Caution
volume of chloroform or dichloromethane and centrifuged to
6.1 Solventsusedinthispracticearevolatile,flammable,or
remove the free water, solids, and debris. The water layer, if
may cause the harm to the health of the user. Specifically,
present, is separated from the organic layer. Other debris, if
benzene is a known carcinogen, while chloroform and carbon
present, is removed by filtration through glass wool.
tetrachloride are suspected carcinogens. Consequently, it is
NOTE 3—The use of spectrograde cyclohexane is required for the
important that extractions and separations utilizing these sub-
extractionofsamplestobeanalyzedbyfluorescencespectrometrybyTest
stances must be carried out in a laboratory hood with a
Method D3650. Separation of water may be accomplished by centrifuga-
minimum linear face velocity of 38 to 45 m/min (125 to 150
tion or dying, or both, with anhydrous sodium sulfate.
ft/min)locatedinaregulatedareapostedwithsignsbearingthe
9.3 Whencentrifugationwillnotseparatethewaterfromthe
legends: NO SMOKING or (if appropriate) DANGER-
chloroform solution of the sample, it is refluxed with an
CHEMICAL CARCINOGEN-AUTHORIZED PERSONNEL
aromaticorpetroleumdistillatesolventinaccordancewithTest
ONLY, or both.
Method D95.
7. Sampling
NOTE 4—Pressure filtration has also been found useful for breaking
emulsions.
7.1 Collect representative samples in accordance with Prac-
tices D4489.
9.4 Aportionofthesolvent/samplesolutionisretained.The
solvent may be removed by evaporation. This portion of the
7.2 Preserve the waterborne oil samples in accordance with
sample may be used in the preliminary gas chromatographic
Practice D3325.
analysis, Test Methods D3328 (Test Method A), and other
7.3 Theportionofthesampleusedmustberepresentativeof
analyses in which the results are unaffected by weathering.
the total sample. If the material is liquid, thoroughly stir the
sampleasreceived,warmingifnecessarytoensureuniformity. 9.5 Theremainderofthesolvent/samplesolutionisdistilled
using nitrogen purge to a liquid temperature of 280°C to
PROCEDURE A—LARGE SAMPLES
remove the solvent and simulate weathering conditions as
nearlyaspossible.Thedistillatemaybediscardedorsavedfor
8. Scope
characterization by gas chromatography (Test Methods
8.1 This procedure covers the preparation for analysis of
D3328). This simulated weathering treatment is necessary to
samples in which the volumes of waterborne oil in the
bring the unweathered suspect samples and the waterborne oil
environmental and suspect source samples equal or exceed 50
sample to as nearly comparable physical condition for subse-
mLandinwhichtheoilportioncontainssignificantamountsof
quent analysis as possible. Analyses requiring the use of this
hydrocarbons with boiling points above 280°C.
treated residue include elemental analysis; gas chromato-
graphicanalysis(TestMethodsD3328,TestMethodsAandB);
aninfraredprocedure(TestMethodD3414);afluorescencetest
Reagent Chemicals, American Chemical Society Specifications, American
method (Test Method D3650); and any applicable test method
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
listed by the American Chemical Society, see Analar Standards for Laboratory or practice described in Practice D3415.
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, NOTE 5—The distillate might yield useful information but is discarded
MD. in this practice.
D3326−07 (2011)
10. Apparatus 11.3 Solvent—Chloroform (stabilized with ethanol) or di-
chloromethane is used for dissolution of the waterborne oil
10.1 Centrifuge, capable of whirling two or more filled
samples. If water is to be removed by distillation, an aromatic,
100-mLcentrifuge tubes at a speed that is controlled to give a
petroleum distillate, or volatile spirits solvent is required as
relative centrifugal force (rcf) between 500 and 800 at the tip
specified in Test Method D95. The safety precautions associ-
of the tubes.
ated with the use of the solvent selected should be considered
10.2 Centrifuge Tubes, cone shaped, 100 mL.
before it is used (see Note 3).
10.3 Distillation Apparatus for Water Determination, as
specified in Test Method D95.
12. Procedure
10.4 Distillation Apparatus for Simulated Weathering, as
12.1 Retention of Neat Samples:
described in Specification E133 except fitted with nitrogen-
12.1.1 Decantorsiphonoffaportionoftheneatwaterborne
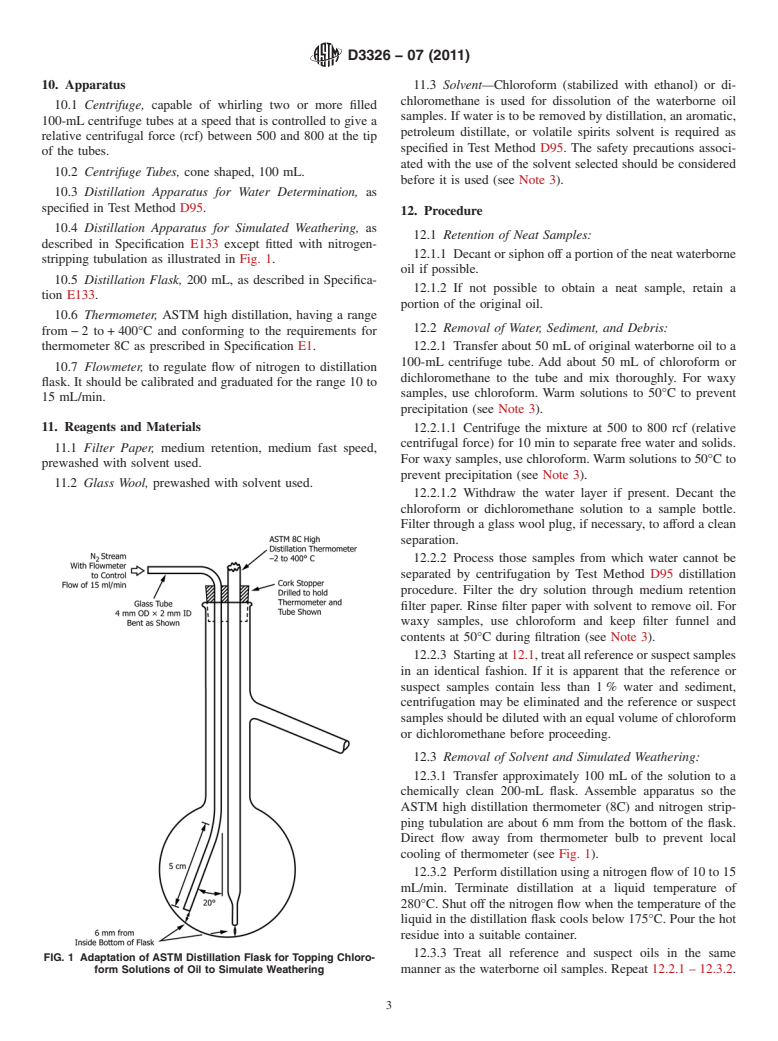
stripping tubulation as illustrated in Fig. 1.
oil if possible.
10.5 Distillation Flask, 200 mL, as described in Specifica-
12.1.2 If not possible to obtain a neat sample, retain a
tion E133.
portion of the original oil.
10.6 Thermometer, ASTM high distillation, having a range
12.2 Removal of Water, Sediment, and Debris:
from−2 to+400°C and conforming to the requirements for
thermometer 8C as prescribed in Specification E1. 12.2.1 Transfer about 50 mLof original waterborne oil to a
100-mL centrifuge tube. Add about 50 mL of chloroform or
10.7 Flowmeter, to regulate flow of nitrogen to distillation
dichloromethane to the tube and mix thoroughly. For waxy
flask. It should be calibrated and graduated for the range 10 to
samples, use chloroform. Warm solutions to 50°C to prevent
15 mL/min.
precipitation (see Note 3).
11. Reagents and Materials
12.2.1.1 Centrifuge the mixture at 500 to 800 rcf (relative
centrifugal force) for 10 min to separate free water and solids.
11.1 Filter Paper, medium retention, medium fast speed,
For waxy samples, use chloroform.Warm solutions to 50°C to
prewashed with solvent used.
prevent precipitation (see Note 3).
11.2 Glass Wool, prewashed with solvent used.
12.2.1.2 Withdraw the water layer if present. Decant the
chloroform or dichloromethane solution to a sample bottle.
Filter through a glass wool plug, if necessary, to afford a clean
separation.
12.2.2 Process those samples from which water cannot be
separated by centrifugation by Test Method D95 distillation
procedure. Filter the dry solution through medium retention
filter paper. Rinse filter paper with solvent to remove oil. For
waxy samples, use chloroform and keep filter funnel and
contents at 50°C during filtration (see Note 3).
12.2.3 Startingat12.1,treatallreferenceorsuspectsamples
in an identical fashion. If it is apparent that the reference or
suspect samples contain less than 1% water and sediment,
centrifugation may be eliminated and the reference or suspect
samplesshouldbedilutedwithanequalvolumeofchloroform
or dichloromethane before proceeding.
12.3 Removal of Solvent and Simulated Weathering:
12.3.1 Transfer approximately 100 mL of the solution to a
chemically clean 200-mL flask. Assemble apparatus so the
ASTM high distillation thermometer (8C) and nitrogen strip-
ping tubulation are about 6 mm from the bottom of the flask.
Direct flow away from thermometer bulb to prevent local
cooling of thermometer (see Fig. 1).
12.3.2 Performdistillationusinganitrogenflowof10to15
mL/min. Terminate distillation at a liquid temperature of
280°C. Shut off the nitrogen flow when the temperature of the
liquid in the distillation flask cools below 175°C. Pour the hot
residue into a suitable container.
12.3.3 Treat all reference and suspect oils in the same
FIG. 1 Adaptation of ASTM Distillation Flask for Topping Chloro-
form Solutions of Oil to Simulate Weathering manner as the waterborne oil samples. Repeat 12.2.1 – 12.3.2.
D3326−07 (2011)
PROCEDURE B—LIMITED SAMPLE VOLUMES OF solvent removal. The samples can then be used for analysis in
HEAVY OILS accordance with Practice D3415.
NOTE 6—This treatment with 70 mg of oil, evaporated at 40°C for 15
13. Scope
min in the presence of an airstream, yielded gas chromatograms resem-
13.1 This procedure covers the preparation for analysis of bling those of the distillation test method in 12.3.
waterborne oil samples of petrol
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.