ASTM F2213-06(2011)
(Test Method)Standard Test Method for Measurement of Magnetically Induced Torque on Medical Devices in the Magnetic Resonance Environment
Standard Test Method for Measurement of Magnetically Induced Torque on Medical Devices in the Magnetic Resonance Environment
SIGNIFICANCE AND USE
This test method is one of those required to determine if the presence of a medical device may cause injury during a magnetic resonance examination and in the magnetic resonance environment. Other safety issues which should be addressed include but may not be limited to magnetically induced force (see Test Method F2052) and RF heating (see Test Method F2182). The terms and icons in Practice F2503 should be used to mark the device for safety in the magnetic resonance environment.
If the maximal torque is less than the product of the longest dimension of the medical device and its weight, then the magnetically induced deflection torque is less than the worst case torque on the device due to gravity. For this condition, it is assumed that any risk imposed by the application of the magnetically induced torque is no greater than any risk imposed by normal daily activity in the Earth's gravitational field. This is conservative; it is possible that greater torques would not pose a hazard to the patient.
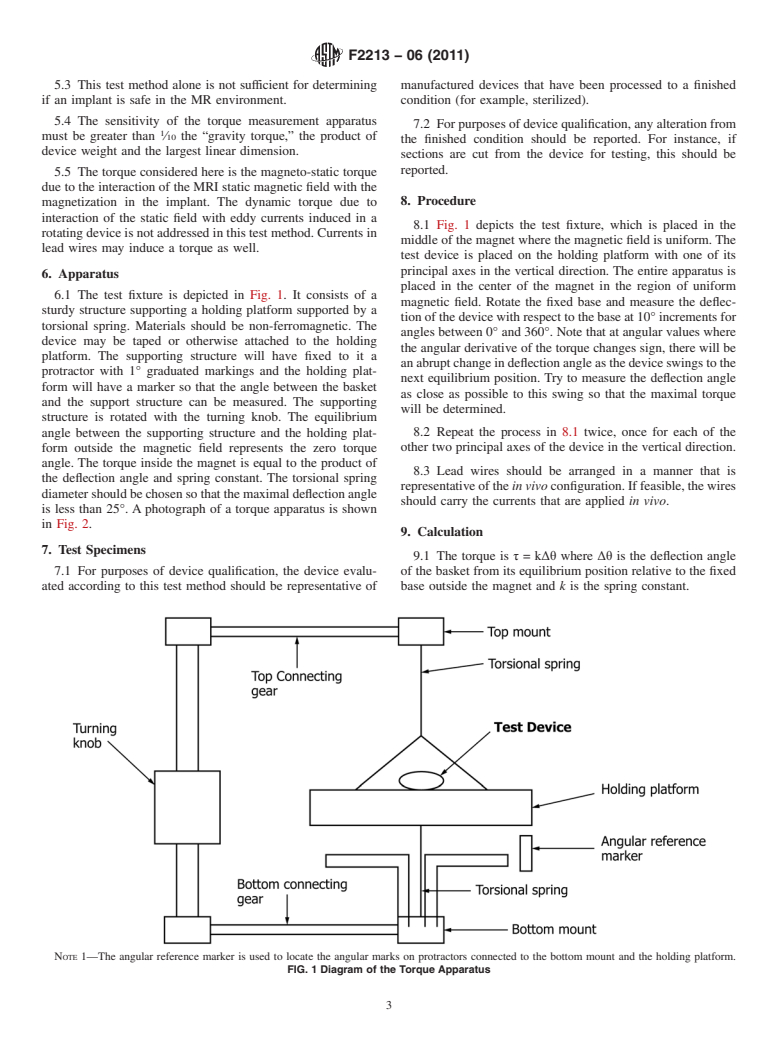
This test method alone is not sufficient for determining if an implant is safe in the MR environment.
The sensitivity of the torque measurement apparatus must be greater than 1/10 the “gravity torque,” the product of device weight and the largest linear dimension.
The torque considered here is the magneto-static torque due to the interaction of the MRI static magnetic field with the magnetization in the implant. The dynamic torque due to interaction of the static field with eddy currents induced in a rotating device is not addressed in this test method. Currents in lead wires may induce a torque as well.
SCOPE
1.1 This test method covers the measurement of the magnetically induced torque produced by the static magnetic field in the magnetic resonance environment on medical devices and the comparison of that torque to the equivalent torque applied by the gravitational force to the implant.
1.2 This test method does not address other possible safety issues which include but are not limited to issues of magnetically induced force due to spatial gradients in the static magnetic field, RF heating, induced heating, acoustic noise, interaction among devices, and the functionality of the device and the MR system.
1.3 The torque considered here is the magneto-static torque due to the interaction of the MRI static magnetic field with the magnetization in the implant. The dynamic torque due to interaction of the static field with eddy currents induced in a rotating device is not addressed in this test method. Currents in lead wires may induce a torque as well.
1.4 The sensitivity of the torque measurement apparatus must be greater than 1/10 the “gravity torque,” the product of the device's maximum linear dimension and its weight.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2213 − 06 (Reapproved 2011)
Standard Test Method for
Measurement of Magnetically Induced Torque on Medical
1
Devices in the Magnetic Resonance Environment
This standard is issued under the fixed designation F2213; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2
1.1 This test method covers the measurement of the mag- 2.1 ASTM Standards:
netically induced torque produced by the static magnetic field F2052Test Method for Measurement of Magnetically In-
inthemagneticresonanceenvironmentonmedicaldevicesand duced Displacement Force on Medical Devices in the
the comparison of that torque to the equivalent torque applied Magnetic Resonance Environment
by the gravitational force to the implant. F2119Test Method for Evaluation of MR Image Artifacts
from Passive Implants
1.2 This test method does not address other possible safety
F2182Test Method for Measurement of Radio Frequency
issues which include but are not limited to issues of magneti-
Induced Heating On or Near Passive Implants During
cally induced force due to spatial gradients in the static
Magnetic Resonance Imaging
magnetic field, RF heating, induced heating, acoustic noise,
F2503Practice for Marking Medical Devices and Other
interaction among devices, and the functionality of the device
Items for Safety in the Magnetic Resonance Environment
and the MR system.
2.2 Other Standards:
1.3 The torque considered here is the magneto-static torque
IEC 60601-2-33Ed. 2.0 Medical Electrical Equipment—
due to the interaction of the MRI static magnetic field with the
Part2:ParticularRequirementsfortheSafetyofMagnetic
3
magnetization in the implant. The dynamic torque due to
Resonance Equipment for Medical Diagnosis, 2002
interaction of the static field with eddy currents induced in a
ISO 13485:2003(E) Medical Devices—Quality Manage-
rotatingdeviceisnotaddressedinthistestmethod.Currentsin
ment Systems—Requirements for Regulatory Purposes,
3
lead wires may induce a torque as well.
definition 3.7
1.4 The sensitivity of the torque measurement apparatus
3. Terminology
1
mustbegreaterthan ⁄10the“gravitytorque,”theproductofthe
3.1 Definitions—For the purposes of this test method, the
device’s maximum linear dimension and its weight.
definitions in 3.1.1 – 3.1.18 shall apply:
1.5 The values stated in SI units are to be regarded as
3.1.1 diamagnetic material—a material whose relative per-
standard. No other units of measurement are included in this
meability is less than unity.
standard.
3.1.2 ferromagnetic material—a material whose magnetic
1.6 This standard does not purport to address all of the
moments are ordered and parallel producing magnetization in
safety concerns, if any, associated with its use. It is the
one direction.
responsibility of the user of this standard to establish appro-
3.1.3 magnetic induction or magnetic flux density (B in
priate safety and health practices and determine the applica-
T)—that magnetic vector quantity which at any point in a
bility of regulatory limitations prior to use.
magnetic field is measured either by the mechanical force
1 2
ThistestmethodisunderthejurisdictionofASTMCommitteeF04onMedical For referenced ASTM standards, visit the ASTM website, www.astm.org, or
andSurgicalMaterialsandDevicesandisthedirectresponsibilityofSubcommittee contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
F04.15 on Material Test Methods. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Oct. 1, 2011. Published October 2011. Originally the ASTM website.
3
approved in 2002. Last previous edition approved in 2006 as F2213–06. DOI: Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
10.1520/F2213-06R11. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2213 − 06 (2011)
experiencedbyanelementofelectriccurrentatthepoint,orby article, intended by the manufacturer to be used, alone or in
the electromotive force induced in an elementary loop during combination, for human beings for one or more of the specific
any change in flux linkages with the loop at the point. The purpose(s) of:
magnetic induction is frequently referred to as the magnetic
(1) diagnosis, prevention, monitoring, treatment, or allevia-
tion of disease,
field. B isthestaticfieldinanMRsystem.Plaintypeindicates
0
(2) diagnosis, monitoring, treatment, alleviation of, or com-
a scalar (
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.