ASTM E476-87(2001)
(Test Method)Standard Test Method for Thermal Instability of Confined Condensed Phase Systems (Confinement Test) (Withdrawn 2008)
Standard Test Method for Thermal Instability of Confined Condensed Phase Systems (Confinement Test) (Withdrawn 2008)
SIGNIFICANCE AND USE
The threshold temperature measured by this test method is an indication of the thermal instability of a chemical or mixture of chemicals, qualitatively expressed by the temperature rise. There is a potential hazard whenever the temperature of the chemical exceeds the threshold temperature unless proper design safeguards are utilized. This does not imply that temperatures lower than the threshold temperature are safe. Since this test is not an adiabatic type and does not indicate the effect of mass or time, other testing would be needed to characterize the use or storage of the chemical at lower temperatures.
Because of rate and mass dependent factors, failure to find evidence of an exothermic reaction does not ensure thermal stability unless substantiated by other test methods.
SCOPE
1.1 This test method is designed to determine the temperature at which a chemical or mixture of chemicals, confined initially as a solid or liquid in air or other controlled atmosphere under normal laboratory conditions, will start a reaction, generating appreciable heat when subjected to a programmed temperature increase. This test method is also designed to measure the magnitude and rate of heat generation.
1.2 This test method is for use with condensed phases.
1.3 This test method can be used over a temperature range from 0 to 500oC, and a pressure range of 0 to 5000 psi.
1.4 As with any thermal stability test, proper safety precautions should be instituted to protect personnel. See also Section 6.
1.5 Limitations
1.5.1 The threshold temperature determined by this method may be higher than one determined by heating at a lesser rate.
1.5.2 Samples of the same material having different thermal histories may have different threshold temperatures.
1.6 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all of the safety problems associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
WITHDRAWN RATIONALE
This test method is designed to determine the temperature at which a chemical or mixture of chemicals, confined initially as a solid or liquid in air or other controlled atmosphere under normal laboratory conditions, will start a reaction, generating appreciable heat when subjected to a programmed temperature increase. This test method is also designed to measure the magnitude and rate of heat generation.
Formerly under the jurisdiction of Committee E27 on Hazard Potential of Chemicals, this test method was withdrawn in November 2008 in accordance with section 10.5.3.1 of the Regulations Governing ASTM Technical Committees, which requires that standards shall be updated by the end of the eighth year since the last approval date.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E 476 – 87 (Reapproved 2001)
Standard Test Method for
Thermal Instability of Confined Condensed Phase Systems
(Confinement Test)
This standard is issued under the fixed designation E 476; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
INTRODUCTION
This test method is one of several methods developed by Committee E-27 for determining the
hazards of chemicals. This test method is to be used in conjunction with other tests to characterize the
hazard potential of chemicals.
1. Scope 2. Terminology
1.1 This test method is designed to determine the tempera- 2.1 threshold temperature—temperature on the DT versus T
ture at which a chemical or mixture of chemicals, confined curve (see Fig. 2) where the slope changes in the direction
initially as a solid or liquid in air or other controlled atmo- indicating an exothermic reaction, that is, the sample is
sphereundernormallaboratoryconditions,willstartareaction, beginning to self-heat.
generating appreciable heat when subjected to a programmed
3. Summary of Method
temperature increase. This test method is also designed to
measure the magnitude and rate of heat generation. 3.1 The sample is confined in a specially designed vessel
equipped with a shielded thermocouple. The test assembly is
1.2 This test method is for use with condensed phases.
1.3 This test method can be used over a temperature range put into a bath and equilibrated, usually at room temperature.
The bath is then heated at a constant temperature rise rate. The
from 0 to 500°C, and a pressure range of 0 to 5000 psi.
1.4 As with any thermal stability test, proper safety precau- differential temperature (sample temperature minus bath tem-
perature) in the vessel is recorded versus bath temperature.
tions should be instituted to protect personnel. See also Section
6. Heating is continued until the diaphragm bursts or the upper
temperaturelimitisreached.Thedifferentialtemperaturecurve
1.5 Limitations:
1.5.1 The threshold temperature determined by this method is then analyzed to determine the threshold temperature for
initiation of measurable reaction as indicated by an exothermic
may be higher than one determined by heating at a lesser rate.
1.5.2 Samples of the same material having different thermal temperature rise.
histories may have different threshold temperatures.
4. Significance and Use
1.6 This standard may involve hazardous materials, opera-
4.1 The threshold temperature measured by this test method
tions, and equipment. This standard does not purport to
is an indication of the thermal instability of a chemical or
address all of the safety problems associated with its use. It is
mixture of chemicals, qualitatively expressed by the tempera-
the responsibility of the user of this standard to establish
ture rise. There is a potential hazard whenever the temperature
appropriate safety and health practices and determine the
of the chemical exceeds the threshold temperature unless
applicability of regulatory limitations prior to use.
proper design safeguards are utilized. This does not imply that
temperatures lower than the threshold temperature are safe.
This test method is under the jurisdiction of CommitteeE27 onHazard Potential
Since this test is not an adiabatic type and does not indicate the
of Chemicals and is the direct responsibility of Subcommittee E 27.02 on Thermal
effect of mass or time, other testing would be needed to
Stability.
characterize the use or storage of the chemical at lower
Current edition approved Sept. 25, 1987. Published November 1987. Originally
e2
published as E 476 – 73. Last previous edition E 476 – 73 (1979) .
temperatures.
This test method is a modification of the Thermal Stability Test recommended
4.2 Because of rate and mass dependent factors, failure to
by the Interagency Chemical Rocket Propulsion Group, published by the Chemical
find evidence of an exothermic reaction does not ensure
Propulsion Information Agency in May, 1964, and is the responsibility of E 27.02
on Thermal Stability. thermal stability unless substantiated by other test methods.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E 476
FIG. 1 Connector, Gasket, and Burst Diaphragm
5.2.1 One recorder is used for recording the difference
between the sample temperature and the bath temperature as a
function of bath temperature.Asuitable recorder for this test is
a standard 8.5 by 11 in. X-Y plotter. Two recorders are required
if pressure versus bath temperature is monitored. Dual-pen
plotters are suitable, provided the temperature pens do not
interact at any critical junction of the reaction.
5.2.2 The maximum reaction rates that can be followed
using the recommended instrumentation are limited by the
writing speeds of the mechanical writing recorders. When
these are calibrated as described in Section 8, rates of reaction
FIG. 2 Idealized Thermogram producingtemperaturechangesof5°Cperscanbedetermined.
Certain reactions may cause temperature changes in excess of
these. If more exact resolution for rapid reactions is desired, it
5. Apparatus
is necessary to use a recording oscillograph in place of the: X-Y
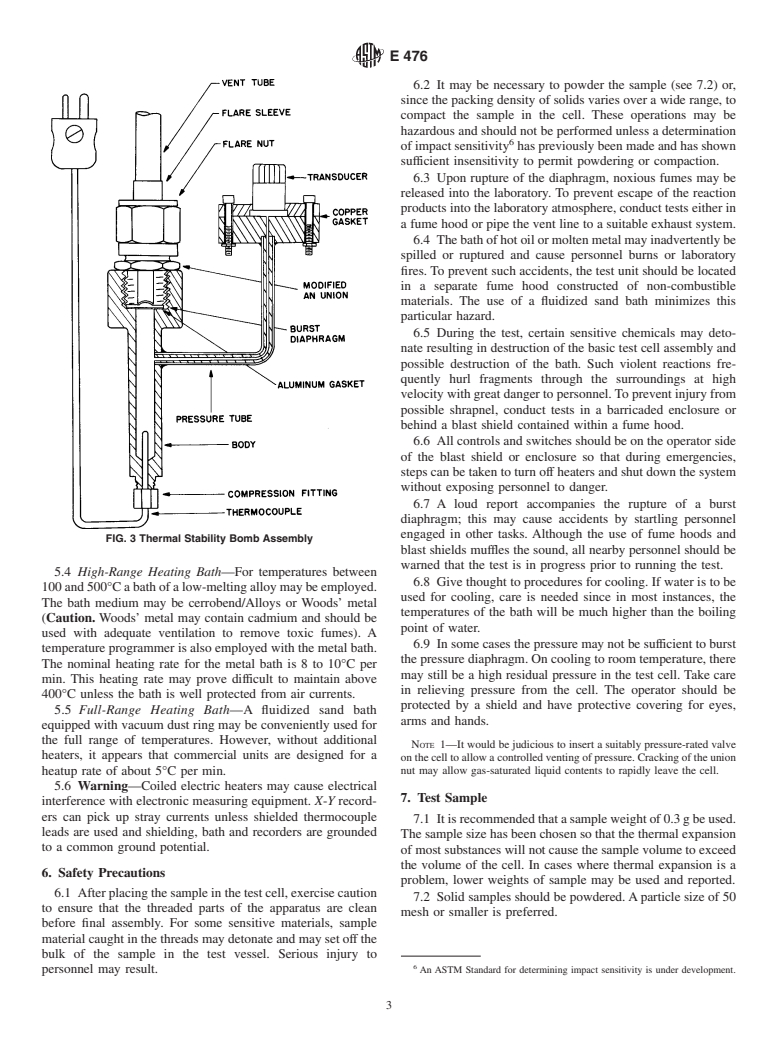
5.1 Sample Container—A diagram of a suggested test cell
recorder.
assembly is shown in Fig. 3 and an engineering drawing is
5.3 Low-Range Heating Bath—For temperatures from 0 to
shown in Fig. 4. The assembly shall consist of the following
370°C the bath may be a conventional 2-Lsilicon oil unit with
parts:basictestcell,samplethermocouple,compressionfitting,
heaters (1800W), stirring motor, and temperature programmer.
sealing ring, burst diaphragm, modified Army-Navy specifica-
The bath container shall be metal with strip heaters on the
tion union (AN union), vent tube and flare fitting. Detailed
outside.Thebathshallbewellinsulated.Acoolingcoilshallbe
dimensions of all parts are given in Fig. 4 with the exception of
wrapped around the container to reduce the time lost between
those parts readily available from manufacturers. The parts are
tests (optional). The coolant shall be tap water. The nominal
listed in Table 1. The internal volume ofthe assembled test cell
heating rate for the bath shall be 8 to 10°C per min.
3,4
is approximately 1.1 mL.
5.2 Instrumentation:
This revised test method, when used with its provision for optional pressure
measurement, is essentially similar to earlier versions of this in which pressure
measurement was an integral part.
3 5
Apparatus of similar designs with a total volume of 1 to 10 mL can be used. Dow Corning 710 has been found satisfactory for this purpose.
E 476
6.2 It may be necessary to powder the sample (see 7.2) or,
since the packing density of solids varies over a wide range, to
compact the sample in the cell. These operations may be
hazardous and should not be performed unless a determination
of impact sensitivity has previously been made and has shown
sufficient insensitivity to permit powdering or compaction.
6.3 Upon rupture of the diaphragm, noxious fumes may be
released into the laboratory. To prevent escape of the reaction
products into the laboratory atmosphere, conduct tests either in
a fume hood or pipe the vent line to a suitable exhaust system.
6.4 Thebathofhotoilormoltenmetalmayinadvertentlybe
spilled or ruptured and cause personnel burns or laboratory
fires. To prevent such accidents, the test unit should be located
in a separate fume hood constructed of non-combustible
materials. The use of a fluidized sand bath minimizes this
particular hazard.
6.5 During the test, certain sensitive chemicals may deto-
nate resulting in destruction of the basic test cell assembly and
possible destruction of the bath. Such violent reactions fre-
quently hurl fragments through the surroundings at high
velocity with great danger to personnel.To prevent injury from
possible shrapnel, conduct tests in a barricaded enclosure or
behind a blast shield contained within a fume hood.
6.6 All controls and switches should be on the operator side
of the blast shield or enclosure so that during emergencies,
steps can be taken to turn off heaters and shut down the system
without exposing personnel to danger.
6.7 A loud report accompanies the rupture of a burst
diaphragm; this may cause accidents by startling personnel
engaged in other tasks. Although the use of fume hoods and
FIG. 3 Thermal Stability Bomb Assembly
blast shields muffles the sound, all nearby personnel should be
warned that the test is in progress prior to running the test.
5.4 High-Range Heating Bath—For temperatures between
6.8 Give thought to procedures for cooling. If water is to be
100and500°Cabathofalow-meltingalloymaybeemployed.
used for cooling, care is needed since in most instances, the
The bath medium may be cerrobend/Alloys or Woods’ metal
temperatures of the bath will be much higher than the boiling
(Caution. Woods’ metal may contain cadmium and should be
point of water.
used with adequate ventilation to remove toxic fumes). A
6.9 In some cases the pressure may not be sufficient to burst
temperature programmer is also employed with the metal bath.
the pressure diaphragm. On cooling to room temperature, there
The nominal heating rate for the metal bath is 8 to 10°C per
may still be a high residual pressure in the test cell. Take care
min. This heating rate may prove difficult to maintain above
in relieving pressure from the cell. The operator should be
400°C unless the bath is well protected from air currents.
protected by a shield and have protective covering for eyes,
5.5 Full-Range Heating Bath—A fluidized sand bath
arms and hands.
equipped with vacuum dust ring
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.