ASTM E2330-04
(Test Method)Standard Test Method for Determination of Trace Elements in Glass Samples Using Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
Standard Test Method for Determination of Trace Elements in Glass Samples Using Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
SIGNIFICANCE AND USE
This technique is destructive, in that the glass fragments may need to be crushed, and must be digested in acid.
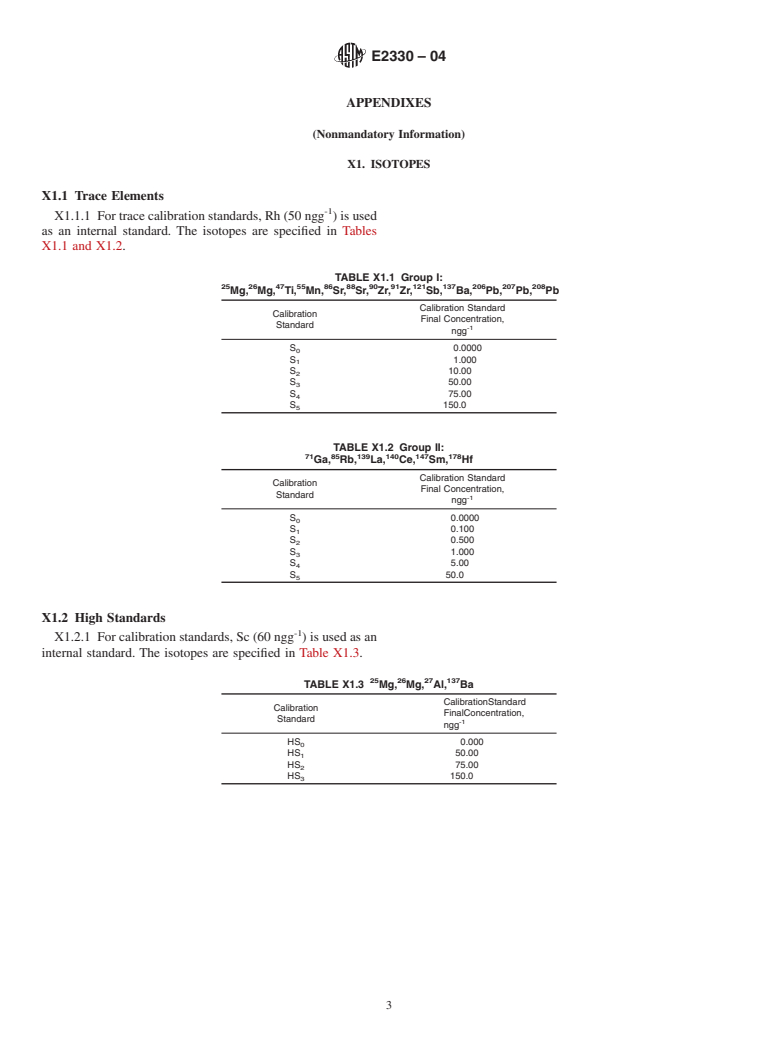
Although the concentration ranges of the calibration curves shown in Appendix X1 are applicable to soda lime and borosilicate glass, this method is useful for the accurate measurement of trace elements from a wide variety of glass samples.
The determination the elemental concentrations in glass allows for additional data that can be compared between fragments. A standardized, validated method can aid in the interchange of data between laboratories.
It should be recognized that the method measures the bulk concentration of the target elements. Any extraneous material present on the glass that is not removed before digestion may result in a measurably different concentration of the elements.
The precision and accuracy of the method should be established in each laboratory that employs the method. Confidence intervals or a similar statistical quality statement should be quoted along with any reported concentration value.
SCOPE
1.1 This test method covers a procedure for elemental analysis of magnesium (Mg), aluminum (Al, titanium (Ti), manganese (Mn), gallium (Ga), rubidium (Rb), strontium (Sr), zirconium (Zr), antimony (Sb), barium (Ba), lanthanum (La), cerium (Ce), samarium (Sm), hafnium (Hf) and lead (Pb) in the bulk of glass samples (irregularly shaped and as small as 200 micrograms) for the comparison of fragments of a known source to the recovered fragments from a questioned source. These elements are present in soda lime and borosilicate glass in ngg-1 to % levels. Alternative methods for the determination of elemental analysis of glass are listed in the references. This procedure may also be applicable to other elements, other types of glass, or other concentration ranges with appropriate modifications of the digestion procedure (if needed for full recovery of the additional elements) and the mass spectrometer conditions. The addition of calcium and potassium, for example, could be added to the list of analytes in a modified analysis scheme.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E2330–04
Standard Test Method for
Determination of Trace Elements in Glass Samples Using
Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
This standard is issued under the fixed designation E2330; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (ϵ) indicates an editorial change since the last revision or reapproval.
1. Scope charged ion interferences.The instrument is then calibrated per
manufacture recommendations using multi-elemental calibra-
1.1 This test method covers a procedure for elemental
tion standards with the same internal standards (Rh and Sc) as
analysis of magnesium (Mg), aluminum (Al, titanium (Ti),
that those added to the samples.
manganese (Mn), gallium (Ga), rubidium (Rb), strontium (Sr),
2.3 Reagent blanks are measured along with the samples as
zirconium (Zr), antimony (Sb), barium (Ba), lanthanum (La),
detection limits are usually limited by the background signals
cerium(Ce),samarium(Sm),hafnium(Hf)andlead(Pb)inthe
generated by the reagent blanks. The limits of detection of the
bulk of glass samples (irregularly shaped and as small as 200
-1 -1
method are expected to be between 0.5 ngg and 25 ngg in
micrograms) for the comparison of fragments of a known
solution for most elements.
source to the recovered fragments from a questioned source.
These elements are present in soda lime and borosilicate glass
3. Significance and Use
-1
in ngg to % levels.Alternative methods for the determination
3.1 This technique is destructive, in that the glass fragments
of elemental analysis of glass are listed in the references. This
may need to be crushed, and must be digested in acid.
proceduremayalsobeapplicabletootherelements,othertypes
3.2 Although the concentration ranges of the calibration
of glass, or other concentration ranges with appropriate modi-
curves shown inAppendix X1 are applicable to soda lime and
fications of the digestion procedure (if needed for full recovery
borosilicate glass, this method is useful for the accurate
of the additional elements) and the mass spectrometer condi-
measurement of trace elements from a wide variety of glass
tions. The addition of calcium and potassium, for example,
samples.
could be added to the list of analytes in a modified analysis
3.3 The determination the elemental concentrations in glass
scheme.
allows for additional data that can be compared between
1.2 This standard does not purport to address all of the
fragments. A standardized, validated method can aid in the
safety concerns, if any, associated with its use. It is the
interchange of data between laboratories.
responsibility of the user of this standard to establish appro-
3.4 It should be recognized that the method measures the
priate safety and health practices and determine the applica-
bulk concentration of the target elements. Any extraneous
bility of regulatory limitations prior to use.
material present on the glass that is not removed before
2. Summary of Test Method digestion may result in a measurably different concentration of
the elements.
2.1 The glass fragments are digested using a mixture of
3.5 The precision and accuracy of the method should be
hydrofluoric, nitric and hydrochloric acids. Following acid
established in each laboratory that employs the method. Con-
digestion,thesamplesaretakentodrynesstoeliminatemostof
fidenceintervalsorasimilarstatisticalqualitystatementshould
the silicate matrix and the excess acids. Then an internal
be quoted along with any reported concentration value.
standardisaddedasthesamplesarereconstitutedinnitricacid.
Dilutions may be utilized to quantitate those elements that are
4. Apparatus
present in higher concentrations.
4.1 ICP-MS—An ICP-MS instrument is employed. Since
2.2 An inductively coupled plasma mass spectrometer is
there are many manufacturers, the specification of the instru-
used to measure the concentrations of the identified elements
ment should be noted within the analysis results.
(1.1). The instrument should be adjusted for maximum sensi-
4.2 The instrument should be tuned prior to the analysis
tivity, best precision and to minimize oxides and doubly
using the manufacturer’s recommendations covering the mass
range of the identified elements. The instrument should be
ThistestmethodisunderthejurisdictionofASTMCommitteeE30onForensic
adjusted for maximum sensitivity, best precision and to mini-
Sciences and is the direct responsibility of Subcommittee E30.01 on Criminalistics.
mize oxides and doubly charged ion interferences. The instru-
Current edition approved Aug. 1, 2004. Published August 2004. DOI: 10.1520/
ment is then calibrated per manufacture recommendations
E2330-04.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E2330–04
-1
using multi-elemental calibration standards with the same with high purity water (>18 MΩ-cm). Wash in 1.6 molL
internal standard as that added to the samples. HNO for 30 min, rinse 3 times with high purity water and
4.3 Replicates—The minimum number of measurement air-dry overnight.
replicates per sample should be three with consideration for
6.2 The samples are crushed between clean polystyrene
additional measurements, if practicable
weighing boats using a pestle, taking care not to puncture the
4.4 Standard Reference Glasses—A minimum of two dif-
boats.
ferent reference glasses of known elemental composition
6.3 Approximately 2 to 3 mg of each sample should be
should be used.
accurately weighed (with a precision of 6 1µg or better) and
quantitativelytransferredintoalabeledpolypropylenetesttube
5. Calibration
with a cap. Each sample should be weighed in triplicate for
5.1 Two calibration curves as well as two check standards
threedeterminationforeachglassexhibit.withaprecisionof6
are used (Appendix X1). The calibration standards must be
1 µg or better.
NIST traceable.
6.4 Allvolumesaredeliveredusingpipetters.Thefollowing
5.2 Forthetraceelementstandardscalibrationcurve,Rh(50
mixture is added to each sample, and standards: 150 µL of
-1
ngg ) is used as an internal standard and the elements are -1
concentrated HNO (16 molL ), 300 µL of concentrated HF
grouped according to the expected concentrations. The first -1 -1
(29 molL ), and 150 µLof concentrated HCl (12 molL ).This
group consists of Mg, Ti, Mn, Sr, Zr, Sb, Ba and Pb with a
acid mixture is also used to prepare the reagent blanks.
-1
concentration range of 0 to 150 ngg . The second group
NOTE 2—All reagents are at least trace metal quality for ICP-MS.
consists of Ga, Rb, La, Ce, Sm and Hf with a concentration
-1
NOTE 3—Normally, the mixture turns pale yellow, and if not, the acid
range of 0 to 50 ngg ppb.
-1
reagentsmayhavelosttheirstrengthandshouldbereplacedbeforeadding
5.3 Thecheckstandardforthecalibrationis50ngg forthe
-1
them to the samples.
first group and 5 ngg for the second group.
-1
5.4 The high standards calibration curve has Sc (60 ngg )
6.5 The tubes are capped, vortex mixed, and placed in an
as an internal standard and is composed of three concentrations
ultrasound bath to assist in the digestion. The tubes are then
-1
levels of Mg, Al, and Ba from 0 to 150 ngg .
uncappedandplacedinadrybathblockoranoven,at80°C(or
5.5 The check standard for the high standard calibration
greater but below the temperature of the softening of the
-1
curve is 60 ngg in all elements including Sc.
digestion tubes), and taken to dryness (about 24 h).
5.6 The system calibration must be checked daily (or on the
6.6 The samples are reconstituted by adding 800- µL of
-1
days the instrument is in use for analysis) and prior to the
HNO (4.0 molL ), 20 µL of an internal standard Rh stock
-1 -1
performance of an analysis, as well as during the analysis after
solution (10 µgg in HNO 0.8 molL ) and 680 µL of high
every ten samples. This is accomplished by the analysis of the
purity water and the tubes are capped.
check standards as a continuing calibration verification (CCV).
6.7 The tubes are vortex mixed and left to stand overnight.
5.7 The system is recalibrated any time that the control falls
6.8 A 2.500 mL volume of high purity water is added, the
outside the acceptable parameters established by the laboratory
tubes are capped and vortex mixed.A50 µLaliquot is removed
or analyst for this procedure (10 % tolerance is recommended).
and the remaining digest solution (undiluted) is analyzed using
5.8 Method Detection Limit (MDL) and Limit of Quantita-
the trace element standardscalibration curve (or curves).
tion (LOQ)—The limits of detection of the method (MDL)are
determined for each element by measuring the three procedure NOTE 4—The 50 µL aliquot of the above solution is transferred to a
polypropylene test tube. A30 µL volume of a scandium (Sc) internal
blanks on two non-consecutive days. Multiply by three the
standard (10 gg-1 in 0.8 molL-1 HNO3) and 4.920 ml of 0.8 molL-1
standard deviation (three instrumental replicates) of the mea-
HNO3 are added. The solution is vigorously mixed before analysis. This
sured intensities calculated by the calibration curve for that
second dilution is analyzed for magnesium, aluminum, and barium with
element in the day that it is measured.To calculate the limits of
the high standards calibration curve.
quantitation for the method (LOQ), multiply by ten the
standard deviation (three instrumental replicates) of the mea-
7. Precision and Bias
sured intensities calculated by the calibration curve for that
7.1 An interlaboratory study was conducted in 2001. Each
elementinthedaythatitismeasured.Themeasuredintensities
offourlaboratoriestestedfourstandardreferenceglassesusing
must be converted to concentration units using the appropriate
5 replicate sample measurements of NIST 612, NIST 614,
calibration curve and the standard deviations calculated from
NIST 621 and NIST 1831.
the concentrations. To calculate these limits of detection and
7.2 The bias and precision results for each of the glasses are
quantitation, the average from the results fo
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.