ASTM D500-95
(Test Method)Standard Test Methods of Chemical Analysis of Sulfonated and Sulfated Oils
Standard Test Methods of Chemical Analysis of Sulfonated and Sulfated Oils
SCOPE
1.1 These test methods cover the chemical analysis of sulfonated and sulfated oils. The analytical procedures appear in the following order: Section Moisture: Test Method A. Water by Distillation with Volatile Solvent 4 to 9 Test Method B. Moisture and Volatile Matter by Hot-Plate Method 10 to 14 Organically Combined Sulfuric Anhydride: Test Method A. Titration Test 15 to 19 Test Method B. Extraction-Titration Test 20 to 24 Test Method C. Ash-Gravimetric Test (in the Presence of True 25 to 28 Sulfonates) Total Desulfated Fatty Matter 29 to 32 Total Active Ingredients 33 to 36 Unsaponifiable Nonvolatile Matter 37 to 41 Inorganic Salts 42 to 46 Total Alkalinity 47 to 49 Total Ammonia 50 to 52 Acidity as Free Fatty Acids or Acid Number: Test Method A. In the Absence of Ammonium or Triethanolamine 53 to 56 Soaps Test Method B. In the Presence of Dark Colored Oils but in the 57 to 60 Absence of Ammonium or Triethanolamine Soaps (Brine Test) Test Method C. In the Presence of Ammonium or Triethanolamine 61 to 63 Soaps Water-Immiscible Organic Solvents Volatile with Steam 64 to 70
1.2 The values stated in inch-pound units are to be re- garded as the standard. The metric equivalents of inch-pound units may be approximate.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Material Safety Data Sheets are available for reagents and materials. Review them for hazards prior to usage.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: D 500 – 95
Standard Test Methods of
Chemical Analysis of Sulfonated and Sulfated Oils
This standard is issued under the fixed designation D 500; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope used in all tests. Unless otherwise indicated, it is intended that
all reagents shall conform to the specifications of the Commit-
1.1 These test methods cover the chemical analysis of
tee on Analytical Reagents of the American Chemical Society,
sulfonated and sulfated oils. The analytical procedures appear
where such specifications are available. Other grades may be
in the following order:
used, provided it is first ascertained that the reagent is of
Section
sufficiently high purity to permit its use without lessening the
Moisture:
accuracy of the determination.
Test Method A. Water by Distillation with Volatile Solvent 4-9
3.2 Purity of Water—Unless otherwise indicated, references
Test Method B. Moisture and Volatile Matter by Hot-Plate Method 10-14
to water shall be understood to mean reagent water conforming
Organically Combined Sulfuric Anhydride:
Test Method A. Titration Test 15-19
to Specification D 1193.
Test Method B. Extraction-Titration Test 20-24
Test Method C. Ash-Gravimetric Test (in the Presence of True 25-28
MOISTURE
Sulfonates)
Method A. Water by Distillation with Volatile Solvent
Total Desulfated Fatty Matter 29-32
Total Active Ingredients 33-36
Unsaponifiable Nonvolatile Matter 37-41
4. Scope
Inorganic Salts 42-46
4.1 This test method covers the determination of water
Total Alkalinity 47-49
Total Ammonia 50-52
existing in a sample of sulfonated or sulfated oil, or both, by
Acidity as Free Fatty Acids or Acid Number:
distilling the sample with a volatile solvent. The method is
Test Method A. In the Absence of Ammonium or Triethanolamine 53-56
applicable only to sulfonated and sulfated oils that do not
Soaps
Test Method B. In the Presence of Dark Colored Oils but in the 57-60
contain the following: mineral acids, free sulfonic acids, or free
Absence of Ammonium or Triethanolamine Soaps (Brine Test)
sulfuric acid esters; or alkali hydroxides, carbonates or ac-
Test Method C. In the Presence of Ammonium or Triethanolamine 61-63
etates; or alcohol, glycerin, diethylene glycol, acetone, or other
Soaps
Water-Immiscible Organic Solvents Volatile with Steam 64-70
water-miscible volatile compounds.
1.2 The values stated in inch-pound units are to be regarded
5. Apparatus
as the standard. The metric equivalents of inch-pound units
5.1 The apparatus required consists of a glass flask heated
may be approximate.
by suitable means and provided with a reflux condenser
1.3 This standard does not purport to address all of the
discharging into a trap and connected to the flask. The
safety concerns, if any, associated with its use. It is the
connections between the trap and the condenser and flask shall
responsibility of the user of this standard to establish appro-
be interchangeable ground joints. The trap serves to collect and
priate safety and health practices and determine the applica-
measure the condensed water and to return the solvent to the
bility of regulatory limitations prior to use. Material Safety
flask. A suitable assembly of the apparatus is illustrated in Fig.
Data Sheets are available for reagents and materials. Review
1.
them for hazards prior to usage.
5.1.1 Flask, 500-mL, of either the short-neck, round-bottom
2. Referenced Documents
type or the Erlenmeyer type.
5.1.2 Heat Source—The source of heat may be either an oil
2.1 ASTM Standards:
bath (stearic acid, paraffin wax, etc.), or an electric heater
D 1193 Specification for Reagent Water
provided with a sliding rheostat or other means of heat control.
3. Purity of Reagents
5.1.3 Condenser—A water-cooled glass reflux condenser
(Fig. 1), having a jacket approximately 400 mm (15 ⁄4 in.) in
3.1 Purity of Reagents—Reagent grade chemicals shall be
These test methods are under the jurisdiction of ASTM Committee D-12 on
Reagent Chemicals, American Chemical Society Specifications, American
Soaps and Other Detergents and is the direct responsibility of Subcommittee D12.12 Chemical Society, Washington, DC. For suggestions on the testing of reagents not
on Analysis of Soaps and Synthetic Detergents. listed by the American Chemical Society, see Analar Standards for Laboratory
Current edition approved April 15, 1995. Published June 1995. Originally Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
published as D 500 – 37. Last previous edition D 500 – 89. and National Formulary, U.S. Pharmaceutical Convention, Inc. (USPC), Rockville,
Annual Book of ASTM Standards, Vol 11.01. MD.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 500
with hot cleaning solution (a mixture of 10 mL of saturated
potassium dichromate (K Cr O ) and 990 mL of sulfuric acid
2 2 7
(H SO , sp gr 1.84)), and finally thoroughly wash and dry.
2 4
8.2 Take enough of the sample to be tested for analysis to
yield about 4 mL of water. Introduce the approximate quantity
into a weighing bottle and make the weighings from the bottle
into the flask, taking care that after removal of the sample no
drops of oil are left on the outside of the weighing bottle. Add
80 g of xylene and oleic acid equivalent to about two and
one-half times the weight of the bone-dry sample to prevent
foaming and jellying of the contents of the flask. Introduce
glass beads to prevent bumping and mix the contents of the
flask thoroughly by swirling, taking care to avoid any loss of
material. Fill the trap with xylene and immediately connect the
flask with the distillation apparatus. Insert a loose cotton plug
in the top of the condenser tube to prevent condensation of
atmospheric moisture in the condenser tube.
8.3 Heat the flask and regulate the heating so that the
condenser tube immediately below the water jacket is just
barely hot. In this way a minimum of water will condense
farther up the condenser where it may be difficult to volatilize
A 5 45 to 55 mm
B 5 22 to 24 mm in inside diameter any moisture condensed on the walls.
C 5 9 to 11 mm in inside diameter
8.4 Continue the distillation at the specified rate until
D 5 235 to 240 mm
practically no water is visible on any part of the apparatus
E 5 146 to 156 mm
F and G are interchangeable joints, standard taper 24/40. except within the graduations of the trap. This operation
usually requires less than 1 h. Increase the rate of distillation in
FIG. 1 Apparatus for Water Determination by Distillation with
order to remove all traces of condensed water in the condenser
Volatile Solvent, Method A
tube, and continue the distillation until the water level in the
trap remains unchanged after a 10-min interval. Dislodge any
3 1
length with an inner tube 9.5 to 12.7 mm ( ⁄8 to ⁄2 in.) in
droplets adhering to the side of the receiver with a thin copper
outside diameter. The end of the condenser to be inserted in the
wire twisted into a loop. Immerse the receiving tube in warm
trap shall be ground off at an angle of 30° from the vertical axis
water at about 40°C for 15 min or until the xylene layer
of the condenser. When inserted into the trap, the tip of the
becomes clear, then read and record the temperature and the
condenser shall be about 7 mm ( ⁄4 in.) above the surface of the
exact volume of the water in the trap.
liquid in the trap after the distillation conditions have been
established. Fig. 1 shows a conventional sealed-in type of
9. Calibration
condenser, but any other condenser fulfilling the detailed
9.1 The volume of condensed water measured in the trap
requirements above may be used.
may be converted into its equivalent weight in grams by means
5.1.4 Trap—A trap made of well-annealed glass constructed
of Table 1. Calculate the percentage of water as follows:
in accordance with Fig. 1 and graduated as shown to contain 5
mL at 20°C. It shall be subdivided into 0.1-mL divisions, with
Water, % 5 ~A/B! 3 100 (1)
each 1-mL line numbered (5 mL at top). The error in any
where:
indicated capacity may not be greater than 0.05 mL.
A 5 weight of water, g, and
6. Reagents B 5 weight of sample, g.
6.1 Oleic Acid, heated previous to use for 5 to 10 min over
a free flame at a temperature of 130 to 135°C.
A
6.2 Xylene. TABLE 1 Specific Gravity of Water
Temperature, °C Specific Gravity
7. Calibration
4 1.00000
7.1 To calibrate the apparatus add approximately1gof
35 0.99406
36 0.99371
water to a mixture of 80 g of xylene and 10 g of oleic acid.
37 0.99336
Conduct the distillation as described in 8.2-8.4. When all the
38 0.99299
water has distilled, cool the apparatus, add another g of water,
39 0.99262
and repeat the distillation. Continue the calibration up to the 40 0.99224
41 0.99186
capacity of the receiving tube.
42 0.99147
43 0.99107
8. Procedure
44 0.99066
45 0.99025
8.1 Clean the condenser and the receiving tube thoroughly
A
with soap and warm water before using. Rinse well, then treat This table is taken from Smithsonian Tables, compiled from various authors.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 500
Method B. Moisture and Volatile Matter by Hot-Plate
A 5 loss of weight, g, and
Method
B 5 weight of sample, g.
10. Scope ORGANICALLY COMBINED SULFURIC
ANHYDRIDE
10.1 This test method covers the determination of the
Method A. Titration Test (For Sulfated Oils)
percentage of water and other compounds volatile at about
100°C existing in a sample of sulfonated or sulfated oil, or
15. Scope
both, by rapid evaporation. The test method is applicable only
15.1 This test method covers the determination of the
to sulfonated and sulfated oils that do not contain the follow-
organically combined sulfuric anhydride existing in a sample
ing: mineral acids, free sulfonic acids or free sulfuric acid
of sulfated oil by boiling the sample with sulfuric acid and
esters, ammonia, acetic acid or similar volatile acids, alkali
determining the acidity of the reaction mixture. This method is
hydroxides, carbonates, acetates or similar salts that may react
applicable only to oils that split off their combined SO upon
with oleic acid at elevated temperatures liberating volatile
boiling with mineral acids and that do not contain compounds
acids, or glycerin, diethylene glycol, xylene, or other com-
that cannot be accurately titrated in water solution with methyl
pounds of similar volatility.
orange as the indicator.
11. Apparatus
16. Apparatus
11.1 The apparatus required consists of a glass-stoppered
16.1 The apparatus required consists of a glass flask pro-
weighing flask, a glass beaker, and a suitable thermometer.
vided with a glass stopper and an air condenser. The connection
11.1.1 Weighing Flasks—Any suitable glass-stoppered
between the flask and the condenser shall be a ground joint.
weighing flask of 10 to 15-mL capacity.
Perforated glass beads shall be used to prevent bumping.
11.1.2 Beaker—A Griffin low-form glass beaker with an
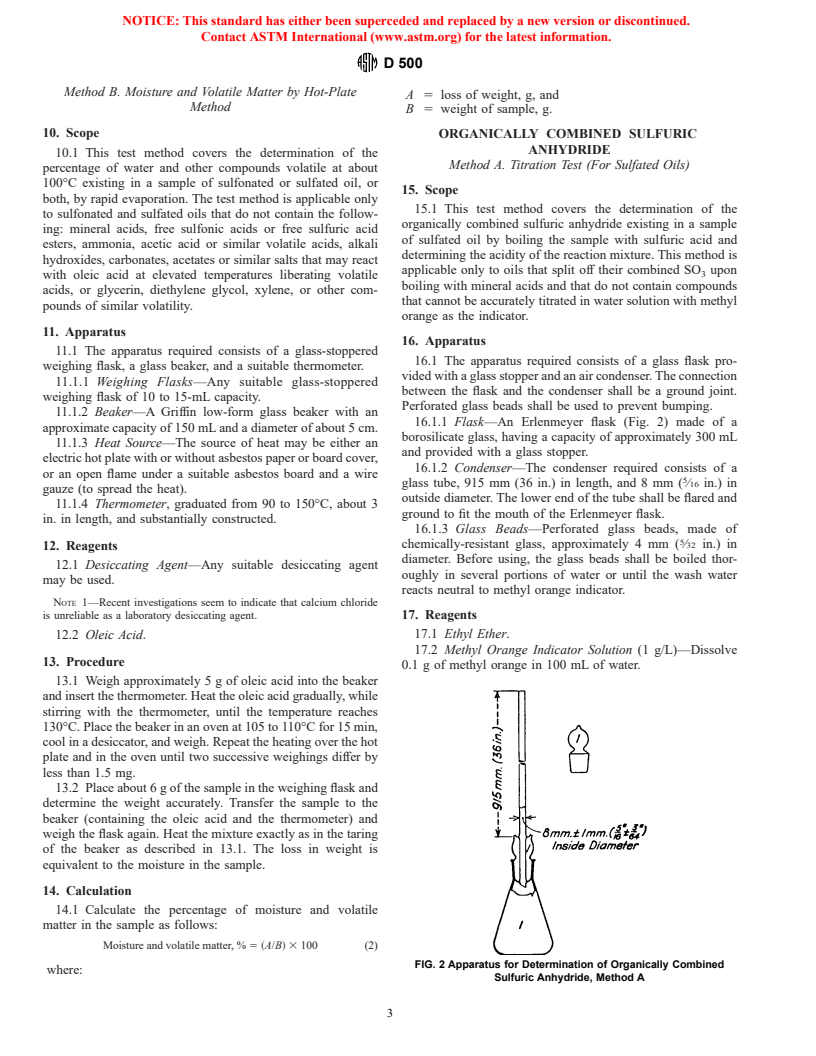
16.1.1 Flask—An Erlenmeyer flask (Fig. 2) made of a
approximate capacity of 150 mL and a diameter of about 5 cm.
borosilicate glass, having a capacity of approximately 300 mL
11.1.3 Heat Source—The source of heat may be either an
and provided with a glass stopper.
electric hot plate with or without asbestos paper or board cover,
16.1.2 Condenser—The condenser required consists of a
or an open flame under a suitable asbestos board and a wire
glass tube, 915 mm (36 in.) in length, and 8 mm ( ⁄16 in.) in
gauze (to spread the heat).
outside diameter. The lower end of the tube shall be flared and
11.1.4 Thermometer, graduated from 90 to 150°C, about 3
ground to fit the mouth of the Erlenmeyer flask.
in. in length, and substantially constructed.
16.1.3 Glass Beads—Perforated glass beads, made of
chemically-resistant glass, approximately 4 mm ( ⁄32 in.) in
12. Reagents
diameter. Before using, the glass beads shall be boiled thor-
12.1 Desiccating Agent—Any suitable desiccating agent
oughly in several portions of water or until the wash water
may be used.
reacts neutral to methyl orange indicator.
NOTE 1—Recent investigations seem to indicate that calcium chloride
is unreliable as a laboratory desiccating agent. 17. Reagents
17.1 Ethyl Ether.
12.2 Oleic Acid.
17.2 Methyl Orange Indicator Solution (1 g/L)—Dissolve
13. Procedure
0.1 g of methyl orange in 100 mL of water.
13.1 Weigh approximately5gof oleic acid into the beaker
and insert the thermometer. Heat the oleic acid gradually, while
stirring with the thermometer, until the temperature reaches
130°C. Place the beaker in an oven at 105 to 110°C for 15 min,
cool in a desiccator, and weigh. Repeat the heating over the hot
plate and in the oven until two successive weighings differ by
less than 1.5 mg.
13.2 Place about6gofthe sample in the weighing flask and
determine the weight accurately. Transfer the sample to the
beaker (containing the oleic acid and the thermometer) and
weigh the flask again. Heat the mixture exactly as in the taring
of the beaker as described in 13.1. The loss in weight is
equivalent to the moisture in the sample.
14. Calculation
14.1 Calculate the percentage of moisture and volatile
matter in the sample as follows:
Moisture and volatile matter, % 5 ~A/B! 3 100 (2)
FIG. 2 Apparatus for Determination of Organically Combined
where:
Sulfuric Anhydride, Method A
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 500
17.3 Sodium Chloride (NaCl), solid. solution with 1 N NaOH solution to the same end point as in
17.4 Sodium Hydroxide, Standard Solution (1 N)— the total alkalinity titration, 18.1.1. During the titration stopper
Accurately prepare and standardize a 1 N sodium hydroxide the flask frequently and shake the contents of the flask
(NaOH) solution. Express the strength or concentration of the thoroughly. Drain the burets for 3 min before readings are
solution as milligrams of KOH per millilitre; 1 mL of 1 N taken.
NaOH solution is equivalent to 56.1 mg of KOH.
NOTE 2—Reserve the titrated solution for the subsequent determination
17.5 So
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.