ASTM D1217-93(1998)
(Test Method)Standard Test Method for Density and Relative Density (Specific Gravity) of Liquids by Bingham Pycnometer
Standard Test Method for Density and Relative Density (Specific Gravity) of Liquids by Bingham Pycnometer
SCOPE
1.1 This test method covers the measurement of the density of pure hydrocarbons or petroleum distillates boiling between 90 and 110°C that can be handled in a normal fashion as a liquid at the specified test temperatures of 20 and 25°C.
1.2 This test method provides a calculation procedure for conversion of density to relative density (specific gravity).
1.3 The values stated in SI units are to be regarded as the standard.
1.4 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in Notes 1, 2, and 3.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn
Contact ASTM International (www.astm.org) for the lastest information
Designation:D1217–93 (Reapproved 1998) An American National Standard
Standard Test Method for
Density and Relative Density (Specific Gravity) of Liquids by
Bingham Pycnometer
This standard is issued under the fixed designation D1217; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope from this weight and the previously determined weight of
water that is required to fill the pycnometer at the same
1.1 This test method covers the measurement of the density
temperature, both weights being corrected for the buoyancy of
of pure hydrocarbons or petroleum distillates boiling between
air.
90 and 110°C that can be handled in a normal fashion as a
liquid at the specified test temperatures of 20 and 25°C.
5. Significance and Use
1.2 This test method provides a calculation procedure for
5.1 Densityisafundamentalphysicalpropertywhichcanbe
conversion of density to relative density (specific gravity).
used in conjunction with other properties to characterize pure
1.3 The values stated in SI units are to be regarded as the
hydrocarbons and their mixtures.
standard.
5.2 This test method was originally developed for the
1.4 This standard does not purport to address all of the
determination of the density of the ASTM Knock Test Refer-
safety concerns, if any, associated with its use. It is the
ence Fuels n-heptane and isooctane, with an accuracy of
responsibility of the user of this standard to establish appro-
0.00003 g/mL.Although it is no longer employed extensively
priate safety and health practices and determine the applica-
for this purpose, this test method is useful whenever accurate
bility of regulatory limitations prior to use. Specific precau-
densities of pure hydrocarbons or petroleum fractions with
tionary statements are given in Note 1, Note 2, and Note 3.
boiling points between 90 and 110°C are required.
2. Referenced Documents
6. Apparatus
2.1 ASTM Standards:
6.1 Pycnometer, Bingham-type, conforming to the dimen-
E1 Specification for ASTM Thermometers
sions given in Fig. 1, constructed of borosilicate glass and
3. Terminology having a total weight not exceeding 30 g.
6.2 Constant-Temperature Bath, provided with suitable py-
3.1 Definitions:
cnometer holders or clips and means for maintaining tempera-
3.1.1 density—the weight in vacuo, (that is, the mass) of a
tures constant to 60.01°C in the desired range.
unit volume of the material at any given temperature.
6.3 Bath Thermometer,graduatedin0.1°Csubdivisionsand
3.1.2 relative density (specific gravity)—the ratio of the
standardizedfortheicepointandtherangeofusetothenearest
mass (weight in vacuo) of a given volume of material at a
0.01°C. ASTM Saybolt Viscosity Thermometer 17C as pre-
temperature, t , to the mass of an equal volume of water at a
scribed in Specification E1, designed for tests at 21.1°C and
reference temperature, t ; or it is the ratio of the density of the
25°C, is recommended. A standardized platinum resistance
material at t to the density of water at t . When the reference
1 2
thermometer may also be used, and offers the best means for
temperature is 4.00°C, the temperature at which the relative
observing minute temperature changes in the bath. Whichever
density of water is unity, relative density (specific gravity) and
means are available, it must be realized that for most hydro-
density are numerically equal.
carbons the density coefficient is about 0.0008 units/°C, and
4. Summary of Test Method
therefore an error of 60.013°C would cause an error of
60.00001 in density.
4.1 The liquid sample is introduced into a pycnometer,
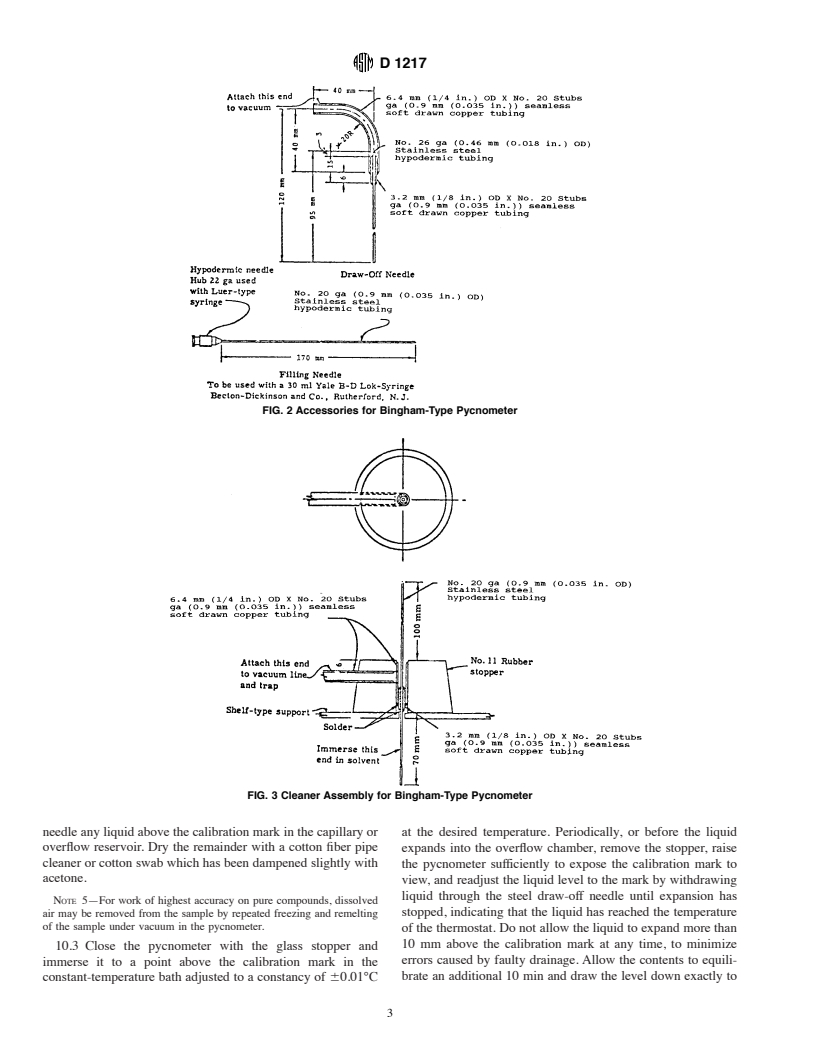
6.4 Hypodermic Syringe, 30-mL capacity, of chemically
equilibrated to the desired temperature, and weighed. The
resistantglass,equippedwitha152-mm(6-in.)needlemadeof
relative density (specific gravity) or density is then calculated
stainless steel tubing as shown in Fig. 2.
6.5 Draw-Off Needle, made of stainless steel tubing as
This test method is under the jurisdiction of ASTM Committee D-2 on
shown in Fig. 2.
Petroleum Products and Lubricantsand is the direct responsibility of Subcommittee
D02.04on Hydrocarbon Analysis.
Current edition approved Feb. 15, 1993. Published May 1993. Originally
published as D 1217–52T. Last previous edition D 1217–91. PycnometeravailablefromRelianceGlassCo.,220GatewayRd.,Bensenville,
Annual Book of ASTM Standards, Vol 14.03. IL 60106-0825, has been found satisfactory.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
D1217
7.3 Chromic Acid (Potassium Dichromate/Conc. Sulfuric
Acid)—(Warning—See Note 3).
NOTE 3—Warning:Causes severe burns.Arecognized carcinogen. Do
not get in eyes, or on skin or clothing.
8. Preparation of Apparatus
8.1 Thoroughlycleanthepycnometerwithhotchromicacid
cleaning solution by means of the assembly shown in Fig. 4
(Warning—See Note 3). Chromic acid solution is the most
effective cleaning agent. However, surfactant cleaning fluids
have also been used successfully. Mount the apparatus firmly
and connect the trap to the vacuum. Warm the necessary
amountofcleaningacidinthebeaker,placethepycnometeron
the ground joint, and evacuate by opening the stopcock to
vacuum.Fillthepycnometerwithacidbyturningthestopcock,
repeatseveraltimesorremovethefilledpycnometer,andallow
it to stand for several hours at 50 to 60°C. Remove the acid
from the pycnometer by evacuation, empty the acid from the
trap, and flush the pycnometer with water. Cleaning should be
made in this manner whenever the pycnometer is to be
calibrated or whenever liquid fails to drain cleanly from the
walls of the pycnometer or its capillary. Ordinarily, the
pycnometer may be cleaned between determinations by wash-
ing with a suitable solvent, rinsing with pure, dry acetone,
followed by isopentane, and vacuum drying.
8.2 Transfer the pycnometer to the cleaner assembly shown
in Fig. 3, with vacuum line and trap attached to the side tube
as indicated. Place the pycnometer on the cleaner with the
upper hypodermic needle extending upward into the pycnom-
FIG. 1 Bingham-Type Pycnometer, 25 mL eter, and press the edge of the ground joint on the rubber
stopper until the vacuum holds it in place. Draw out all the
6.6 Solvent-Cleaning Assembly, as shown in Fig. 3.
liquid or sample. Immerse the lower end of the hypodermic
6.7 Chromic Acid Cleaning Apparatus, similar to that
tube in a suitable solvent and draw 20 to 25 mL through the
shown in Fig. 4.
pycnometer.Leavingthepycnometerinplace,drawairthrough
6.8 Balance, capable of reproducing weighings within 0.1
it until it is dry. Clean the hypodermic syringe with the same
mg. Mechanical balances should have sensitivity which causes
apparatus.
thepointertobedeflected2or3scaledivisionsper1mgwhen
9. Calibration of Pycnometer
carryingaloadof30gorlessoneachpan.Thebalanceshould
be located in a room shielded from drafts and fumes and in
9.1 Proceeding as directed in Section 10, determine the
which the temperature changes between related weighings
weight of freshly-boiled and cooled distilled water (distilled
(empty and filled pycnometer) do not cause a significant
from alkaline permanganate through a tin condenser) held by
change in the ratio of the balance arms. Otherwise weighings
the pycnometer when equilibrated to volume at the bath
shall be made by the method of substitution, in which the
temperature to be used in the determination. Repeat until at
calibrated weights and pycnometer are alternately weighed on
least three values agree to 60.2 mg.
the same balance pan. The same balance shall be used for all
related weighings. 10. Procedure
6.9 Weights, whose relative values are known to the nearest
10.1 Using another 25-mL pycnometer as a tare (Note 4),
0.05mgorbetter.Thesamesetofweightsshallbeusedforthe
weigh the clean, dry pycnometer to 0.1 mg and record the
calibration of the pycnometer and the determination of densi-
weight.
ties.
NOTE 4—It is convenient to use the lightest of a set of pycnometers as
7. Reagents and Materials a tare. For best results the treatment and environment of both pycnometer
and tare should be identical for some time prior to weighing.
7.1 Acetone—(Warning—See Note 1).
10.2 Cool the sample to 5 to 10°C below the test tempera-
NOTE 1—Warning:Extremely flammable. Use adequate ventilation.
ture,andfilltheclean30-mLhypodermicsyringe.Transferthe
7.2 Isopentane—( Warning—See Note 2).
sample to the pycnometer through the filling needle; avoid
trapping air bubbles (Note 2) in the bulb
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.