ASTM G102-89(2010)
(Practice)Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements
Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements
SIGNIFICANCE AND USE
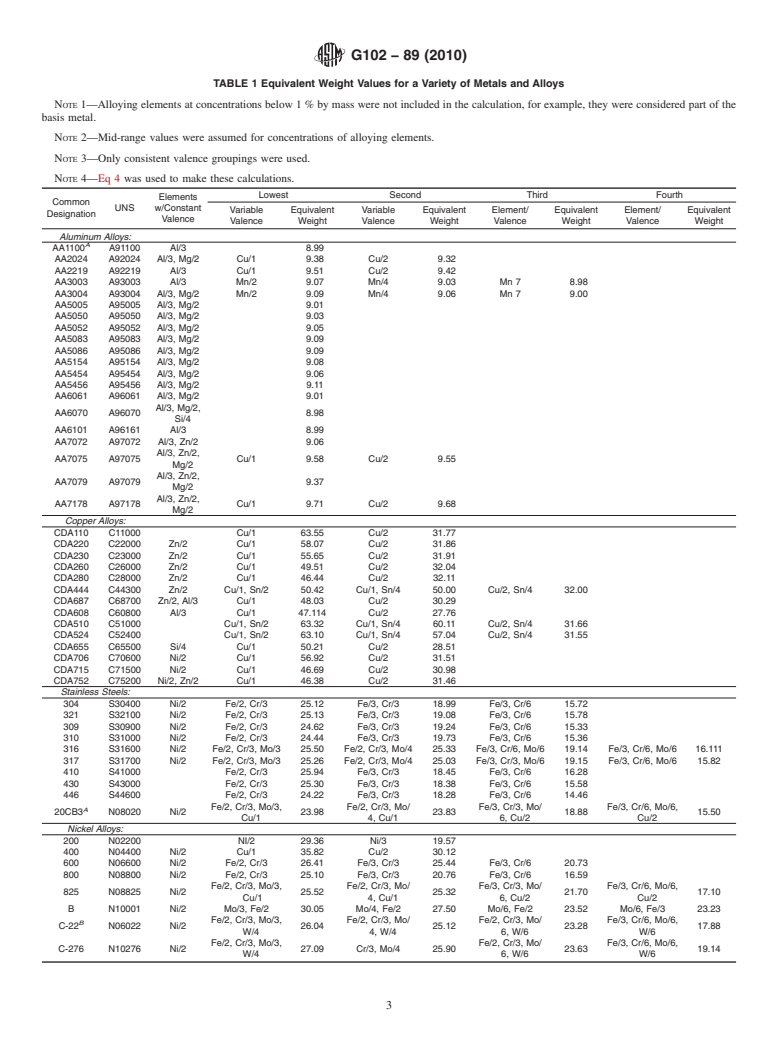
Electrochemical corrosion rate measurements often provide results in terms of electrical current. Although the conversion of these current values into mass loss rates or penetration rates is based on Faraday's Law, the calculations can be complicated for alloys and metals with elements having multiple valence values. This practice is intended to provide guidance in calculating mass loss and penetration rates for such alloys. Some typical values of equivalent weights for a variety of metals and alloys are provided.
Electrochemical corrosion rate measurements may provide results in terms of electrical resistance. The conversion of these results to either mass loss or penetration rates requires additional electrochemical information. Some approaches for estimating this information are given.
Use of this practice will aid in producing more consistent corrosion rate data from electrochemical results. This will make results from different studies more comparable and minimize calculation errors that may occur in transforming electrochemical results to corrosion rate values.
SCOPE
1.1 This practice covers the providing of guidance in converting the results of electrochemical measurements to rates of uniform corrosion. Calculation methods for converting corrosion current density values to either mass loss rates or average penetration rates are given for most engineering alloys. In addition, some guidelines for converting polarization resistance values to corrosion rates are provided.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: G102 − 89(Reapproved 2010)
Standard Practice for
Calculation of Corrosion Rates and Related Information
1
from Electrochemical Measurements
This standard is issued under the fixed designation G102; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope multiple valence values. This practice is intended to provide
guidanceincalculatingmasslossandpenetrationratesforsuch
1.1 This practice covers the providing of guidance in
alloys. Some typical values of equivalent weights for a variety
convertingtheresultsofelectrochemicalmeasurementstorates
of metals and alloys are provided.
of uniform corrosion. Calculation methods for converting
corrosion current density values to either mass loss rates or
3.2 Electrochemical corrosion rate measurements may pro-
averagepenetrationratesaregivenformostengineeringalloys.
vide results in terms of electrical resistance.The conversion of
In addition, some guidelines for converting polarization resis-
these results to either mass loss or penetration rates requires
tance values to corrosion rates are provided.
additional electrochemical information. Some approaches for
estimating this information are given.
1.2 The values stated in SI units are to be regarded as
standard. No other units of measurement are included in this
3.3 Use of this practice will aid in producing more consis-
standard.
tent corrosion rate data from electrochemical results. This will
make results from different studies more comparable and
2. Referenced Documents
minimize calculation errors that may occur in transforming
2
2.1 ASTM Standards:
electrochemical results to corrosion rate values.
D2776Methods of Test for Corrosivity of Water in the
Absence of Heat Transfer (Electrical Methods) (With-
4. Corrosion Current Density
3
drawn 1991)
4.1 Corrosioncurrentvaluesmaybeobtainedfromgalvanic
G1Practice for Preparing, Cleaning, and Evaluating Corro-
cells and polarization measurements, including Tafel extrapo-
sion Test Specimens
lations or polarization resistance measurements. (See Refer-
G5Reference Test Method for Making Potentiodynamic
enceTestMethodG5andPracticeG59forexamples.)Thefirst
Anodic Polarization Measurements
step is to convert the measured or estimated current value to
G59TestMethodforConductingPotentiodynamicPolariza-
current density. This is accomplished by dividing the total
tion Resistance Measurements
current by the geometric area of the electrode exposed to the
3. Significance and Use
solution. The surface roughness is generally not taken into
accountwhencalculatingthecurrentdensity.Itisassumedthat
3.1 Electrochemicalcorrosionratemeasurementsoftenpro-
the current distributes uniformly across the area used in this
vide results in terms of electrical current. Although the con-
calculation.Inthecaseofgalvaniccouples,theexposedareaof
version of these current values into mass loss rates or penetra-
the anodic specimen should be used. This calculation may be
tion rates is based on Faraday’s Law, the calculations can be
expressed as follows:
complicated for alloys and metals with elements having
I
cor
i 5 (1)
cor
A
1
This practice is under the jurisdiction ofASTM Committee G01 on Corrosion
of Metalsand is the direct responsibility of Subcommittee G01.11 on Electrochemi-
where:
cal Measurements in Corrosion Testing.
2
I = corrosion current density, µA/cm ,
Current edition approved May 1, 2010. Published May 2010. Originally
cor
ε1
approved in 1989. Last previous edition approved in 2004 as G102–89(2004) .
I = total anodic current, µA, and
cor
2
DOI: 10.1520/G0102-89R10.
A = exposed specimen area, cm .
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Other units may be used in this calculation. In some
Standards volume information, refer to the standard’s Document Summary page on
computerized polarization equipment, this calculation is made
the ASTM website.
3 automatically after the specimen area is programmed into the
The last approved version of this historical standard is referenced on
www.astm.org. computer. A sample calculation is given in Appendix X1.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
G102 − 89 (2010)
4.2 Equivalent Weight—Equivalent weight, EW, may be namic data. These diagrams are known as Potential-pH (Pour-
thought of as the mass of metal in grams that will be oxidized baix) diagrams and have been published by several authors (2,
by the
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.