ASTM D3414-98
(Test Method)Standard Test Method for Comparison of Waterborne Petroleum Oils by Infrared Spectroscopy
Standard Test Method for Comparison of Waterborne Petroleum Oils by Infrared Spectroscopy
SCOPE
1.1 This test method provides a means for the identification of waterborne oil samples by the comparison of their infrared spectra with those of potential source oils.
1.2 This test method is applicable to weathered or unweathered samples, as well as to samples subjected to simulated weathering.
1.3 This test method is written primarily for petroleum oils.
1.4 This test method is written for linear transmission, but could be readily adapted for linear absorbance outputs.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in Section 8.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation: D 3414 – 98
Standard Test Method for
Comparison of Waterborne Petroleum Oils by Infrared
Spectroscopy
This standard is issued under the fixed designation D 3414; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Terminology
1.1 This test method provides a means for the identification 3.1 Definitions:
of waterborne oil samples by the comparison of their infrared 3.1.1 For definitions of terms used in this test method refer
spectra with those of potential source oils. to Terminology E 131 and Terminology D 1129.
1.2 This test method is applicable to weathered or unweath- 3.2 Definitions of Terms Specific to This Standard:
ered samples, as well as to samples subjected to simulated 3.2.1 weathering of waterborne oil—the combined effects
weathering. of evaporation, solution, emulsification, oxidation, biological
1.3 This test method is written primarily for petroleum oils. decomposition, etc.
1.4 This test method is written for linear transmission, but
4. Summary of Test Method
could be readily adapted for linear absorbance outputs.
4.1 The spill sample and potential source oil(s) are treated
1.5 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the identically to put them in an appropriate form for analysis by
infrared spectrophotometry. The oils are transferred to suitable
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- infrared cells and the spectra are recorded from 4000 to 600
−1
cm for KBr cells, and to 650 cm-1 for HATR cells with ZnSe
bility of regulatory limitations prior to use. Specific precau-
tionary statements are given in Section 8. crystals. All analyses are performed on the same instrument
using the same sample cell, which is cleaned between samples.
2. Referenced Documents
The spectra of the sample and the potential source oil(s) are
2.1 ASTM Standards: then compared by superimposing one upon the other, looking
D 1129 Terminology Relating to Water at particular portions of the spectra. A high degree of coinci-
D 1193 Specification for Reagent Water dence between the spectra of the sample and a potential source
D 3325 Practice for Preservation of Waterborne Oil oil indicates a common origin. This test method is recom-
Samples mended for use by spectroscopists experienced in infrared oil
D 3326 Practice for Preparation of Samples for Identifica- identification or under close supervision of such qualified
tion of Waterborne Oils persons.
D 3415 Practice for Identification of Waterborne Oils
4 5. Significance and Use
E 131 Terminology Relating to Molecular Spectroscopy
E 168 Practices for General Techniques of Infrared Quan- 5.1 This test method provides a means for the comparison of
waterborne oil samples with potential sources. The waterborne
titative Analysis
E 275 Practice for Describing and Measuring Performance samples may be emulsified in water or obtained from beaches,
boats, oil-soaked debris, etc.
of Ultraviolet, Visible, and Near Infrared Spectrophotom-
eters 5.2 The unknown oil is identified by the similarity of its
infrared spectrum with that of a potential source oil obtained
from a known source, selected because of its possible relation-
ship to the unknown oil.
This test method is under the jurisdiction of ASTM Committee D19 on Water
5.3 The analysis is capable of comparing most oils. Diffi-
and is the direct responsibility of Subcommittee D19.06 on Methods for Analysis for
culties may be encountered if a spill occurs in an already
Organic Substances in Water.
Current edition approved July 10, 1998. Published December 1998. Originally
polluted area, that is, the spilled-oil mixes with another oil.
published as D 3414–75 T. Last previous edition D 3414–80 (1990).
5.4 In certain cases, there may be interfering substances
Annual Book of ASTM Standards, Vol 11.01.
3 which require modification of the infrared test method or the
Annual Book of ASTM Standards, Vol 11.02.
Annual Book of ASTM Standards, Vol 03.06. use of other test methods (see Practice D 3326, Method D.)
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 3414 – 98
TABLE 1 Specifications for Infrared Spectrophotometers
6.4.2 Disposable Pasteur Pipets and Hypodermic Syringes.
−1
Abscissa accuracy Better than 65cm from 4000 to 2000 6.4.3 Window-Polishing Kit.
−1 −1
cm range; better than 63cm
6.4.4 Centrifuge.
−1
from 2000 to 600 cm (or below).
−1 −1 6.4.5 Vortex Mixer.
Abscissa repeatability 2.5 cm from 4000 to 2000 cm ; 1.5
−1 −1
cm from 2000 to 600 cm (or below). 6.4.6 Hot Plate.
Ordinate accuracy 6 1 % of full scale.
6.4.7 Light-Box, for viewing spectra.
Ordinate repeatability within 1 % of full scale.
7. Reagents
7.1 Purity of Reagents—Reagent grade chemicals shall be
5.5 It is desirable, whenever possible, to apply other inde-
used in all tests unless otherwise indicated. It is intended that
pendent analytical test methods to reinforce the findings of the
all reagents shall conform to the specifications of the Commit-
infrared test method (see Practice D 3415).
tee on Analytical Reagents of the American Chemical Society,
where such specifications are available. For sample treatment
6. Apparatus
and for cleaning cells, special spectroquality reagents are
6.1 Infrared Spectrophotometer—An instrument capable
required. Other grades may be used, provided it is first
−1
of recording in the spectral range from 4000 to 600 cm and
established that the reagent is of sufficiently high purity to
meeting the specifications is shown in Table 1. Refer also to
permit its use without decreasing the accuracy of the determi-
Practice E 275. Fourier transform infrared spectrophotometers
nation.
meet these specifications.
7.2 Purity of Water— Unless otherwise indicated references
NOTE 1—Although this test method is written for the use of dispersive
to water shall be understood to mean reagent water conforming
infrared spectroscopy, Fourier transform infrared spectroscopy can also be
to Specification D 1193, Type II.
used for oil comparison.
7.3 Magnesium Sulfate—anhydrous, reagent grade, for dry-
6.2 Sample Cells:
ing samples.
6.2.1 Demountable Cells—The cell generally used is a
7.4 Solvents—Spectroquality solvents for sample treatment
demountable liquid cell using a 0.05-mm spacer. This cell is
and cleaning cells include cyclohexane, pentane, hexane,
usable for all oil types, the heavy oils being analyzed as
methylene chloride, and methanol.
smears. For light oils, a sealed cell can be used, provided that
the sample is known to be dry. Another type used is a 8. Precautions
low-capacity demountable cell using a silver halide window
8.1 Take normal safety precautions when handling organic
with a 0.025-mm depression. Satisfactory oil spectra can be
solvents. Take precautions to ensure that wet oil samples do not
obtained with this cell with as little as 10 μL of oil, compared
come in contact with water-soluble cell window materials.
to the nearly 100 μL required for the standard cells. This cell
Most spectrophotometers require humidity control (to about
can also be used to screen for the presence of water in oil
45 %), particularly if they have humidity-sensitive detectors
samples.
such as those with cesium iodide optics. The primary precau-
6.2.2 Horizontal Attentuated Reflectance Apparatus
tion which must be taken to provide the best possible results is
(HATR), may be used instead of demountable cells. If so, all
that all samples analyzed should be treated in an identical
analyses must be performed with the same HATR apparatus.
fashion, run in the same cell, on the same instrument and
6.3 Cell Windows:
preferably on the same day by the same operator.
6.3.1 Potassium or silver bromide should be used for
NOTE 3—If the samples cannot be analyzed the same day, one of the
demountable cells. Silver chloride may be substituted for the
first samples must be repeated to ensure that the spectra are not
bromide.
significantly different.
NOTE 2—Sodium chloride should not be used; results obtained using
9. Sampling
this window material, although consistent with each other, are not directly
comparable to those from the other window materials. Sodium chloride
9.1 On-Scene—A representative sample of the waterborne
was shown by Brown, et al to give results significantly different from
oil is collected in a glass jar (precleaned with cyclohexane and
those obtained with potassium bromide or silver chloride, based on
dried) having a TFE-fluorocarbon-lined cap. In the same time
quantitative comparisons.
frame, samples are collected of potential source samples that
6.3.2 Zinc selenide is the material of choice for the HATR
are to be compared to the waterborne sample.
apparatus.
9.2 Laboratory—See Annex A1.
6.4 Accessories:
6.4.1 Reference Beam Attenuator, for setting baseline with
10. Preservation of Sample
the low-capacity silver halide cell.
10.1 Refer to Practice D 3325.
Consult the manufacturer’s operating manual for specific instructions on using
this apparatus. “Reagent Chemicals, American Chemical Society Specifications,” American
The Mini-cell made by Wilks Scientific Corp., S. Norwalk, CT, has been found Chemical Society, Washington, DC. For suggestions on the testing of reagents not
to be satisfactory for this purpose. listed by the American Chemical Society, see Rosin, J.,“ Reagent Chemicals and
Brown, C. W., Lynch, P. F., and Ahmadjian, M. “Identification of Oil Slicks by Standards,” D. Van Nostrand Co., New York, NY, and the “United States
Infrared Spectroscopy,” NTIS Accession No. ADA 040975, 1976. Pharmacopeia”.
D 3414 – 98
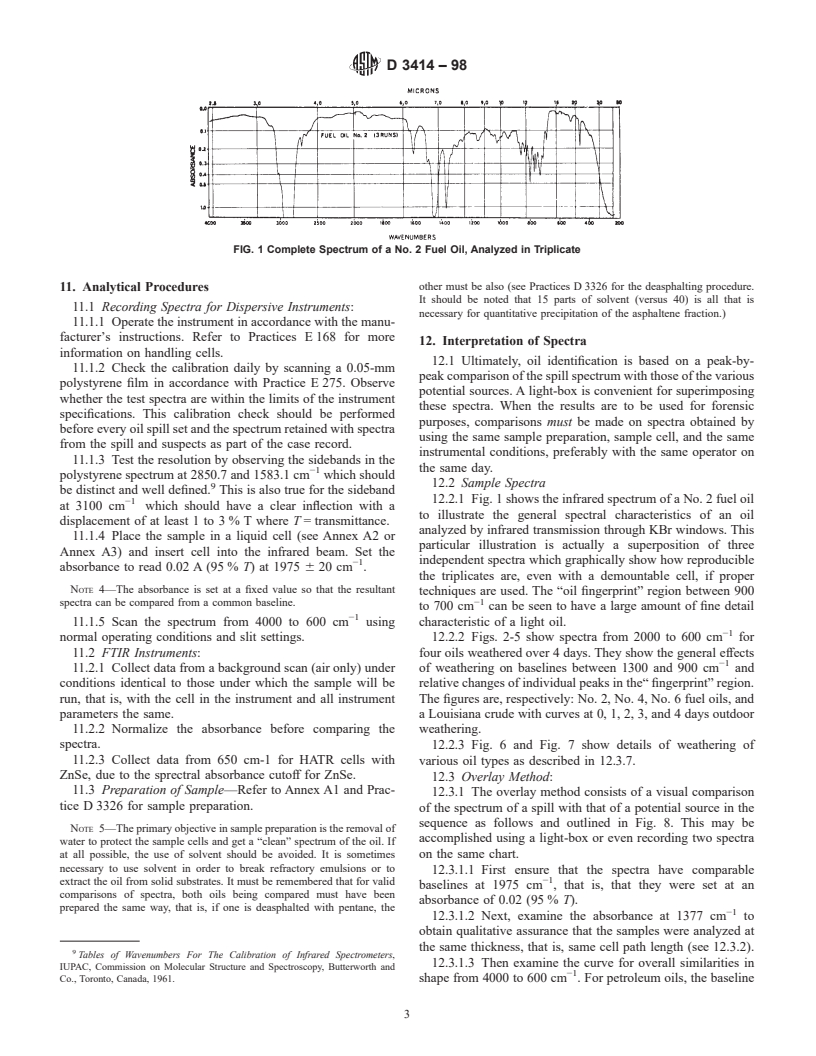
FIG. 1 Complete Spectrum of a No. 2 Fuel Oil, Analyzed in Triplicate
other must be also (see Practices D 3326 for the deasphalting procedure.
11. Analytical Procedures
It should be noted that 15 parts of solvent (versus 40) is all that is
11.1 Recording Spectra for Dispersive Instruments:
necessary for quantitative precipitation of the asphaltene fraction.)
11.1.1 Operate the instrument in accordance with the manu-
facturer’s instructions. Refer to Practices E 168 for more
12. Interpretation of Spectra
information on handling cells.
12.1 Ultimately, oil identification is based on a peak-by-
11.1.2 Check the calibration daily by scanning a 0.05-mm
peak comparison of the spill spectrum with those of the various
polystyrene film in accordance with Practice E 275. Observe
potential sources. A light-box is convenient for superimposing
whether the test spectra are within the limits of the instrument
these spectra. When the results are to be used for forensic
specifications. This calibration check should be performed
purposes, comparisons must be made on spectra obtained by
before every oil spill set and the spectrum retained with spectra
using the same sample preparation, sample cell, and the same
from the spill and suspects as part of the case record.
instrumental conditions, preferably with the same operator on
11.1.3 Test the resolution by observing the sidebands in the
the same day.
−1
polystyrene spectrum at 2850.7 and 1583.1 cm which should
12.2 Sample Spectra
be distinct and well defined. This is also true for the sideband
12.2.1 Fig. 1 shows the infrared spectrum of a No. 2 fuel oil
−1
at 3100 cm which should have a clear inflection with a
to illustrate the general spectral characteristics of an oil
displacement of at least 1 to 3 % T where T = transmittance.
analyzed by infrared transmission through KBr windows. This
11.1.4 Place the sample in a liquid cell (see Annex A2 or
particular illustration is actually a superposition of three
Annex A3) and insert cell into the infrared beam. Set the
−1 independent spectra which graphically show how reproducible
absorbance to read 0.02 A (95 % T) at 1975 6 20 cm .
the triplicates are, even with a demountable cell, if proper
NOTE 4—The absorbance is set at a fixed value so that the resultant
techniques are used. The “oil fingerprint” region between 900
−1
spectra can be compared from a common baseline.
to 700 cm can be seen to have a large amount of fine detail
−1
11.1.5 Scan the spectrum from 4000 to 600 cm using characteristic of a light oil.
−1
normal operating conditions and slit settings. 12.2.2 Figs. 2-5 show spectra from 2000 to 600 cm for
11.2 FTIR Instruments: four oils weathered over 4 days. They show the general effects
−1
11.2.1 Collect data from a background scan (air only) under
of weathering on baselines between 1300 and 900 cm and
conditions identical to those under which the sample will be relative changes of individual peaks in the“ fingerprint” region.
run, that is, with the cell in the instrument and all instrument
The figures are, respectively: No. 2, No. 4, No. 6 fuel oils, and
parameters the same. a Louisiana crude with curves at 0, 1, 2, 3, and 4 days outdoor
11.2.2 Normalize the absorbance before comparing the weathering.
spectra. 12.2.3 Fig. 6 and Fig. 7 show details of weathering of
11.2.3 Collect data from 650 cm-1 for HATR cells with
various oil types as described in 12.3.7.
ZnSe, due to the sprectral absorbance cutoff for ZnSe.
12.3 Overlay Method:
11.3 Preparation of Sample—Refer to Annex A1 and Prac-
12.3.1 The overlay method consists of a visual comparison
tice D 3326 for sample preparation.
of the spectrum of a spill with that of a potential source in the
sequence as follows and outlined in Fig. 8. This may be
NOTE 5—The primary objective in sample preparation is the removal of
accomplished using a light-box or even recording two spectra
water to protect the sample cells and get a “clean” spectrum of the oil. If
at all possible, the use of solvent should be avoided. It is sometimes on the same chart.
necessary to use solvent in order to break refractory emulsions or to
12.3.1.1 First ensure that the spectra have comparable
−1
extract the oil from solid substrates. It must be remembered that for valid
baselines at 1975 cm , that is, that they were set at an
comparisons of spectra, both oils being compared must have been
absorbance of 0.02 (95 % T).
prepared the same way, that is, if one is deasphalted with pentane, the
−1
12.3.1.2 Next, examine the absorbance at 1377 cm to
obtain qualitative assurance that the samples were analyzed at
the same thickness, that is, same cell path length (see 12.3.2).
Tables of Wavenumbers For The Calibration of Infrared Spectrometers,
12.3.1.3 Then
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.