ASTM D6345-98(2004)e1
(Guide)Standard Guide for Selection of Methods for Active, Integrative Sampling of Volatile Organic Compounds in Air
Standard Guide for Selection of Methods for Active, Integrative Sampling of Volatile Organic Compounds in Air
SIGNIFICANCE AND USE
This guide provides a broad perspective on techniques that can be used by environmental managers for selecting VOC air monitoring methods. It summarizes various methods for measurement of VOC in air derived from a variety of sources and experiences and incorporates them into condensed guidelines. This guide provides a common basis for selecting methods for VOC measurement as well a discussion of the limitations of typical methods.
This guide should be used during the planning stages of an air monitoring program along with other applicable guides and practices (for example, D 1357) to select ASTM or other appropriate methods.
SCOPE
1.1 This guide provides assistance in the selection of active integrative sampling methods, in which the volatile organic analytes are collected from air over a period of time by drawing the air into the sampling device, with subsequent recovery for analysis. Where available, specific ASTM test methods and practices are referenced.
1.2 Guidance is provided for the selection of active sampling methods based either on collection of an untreated air sample (whole air samples) or selective sampling using sorbent concentration techniques that selectively concentrate components in air. Advantages and disadvantages of specific collection vehicles are presented.
1.3 This guide does not cover the use of cryogenically cooled field sampling devices used in some automated analysis systems. Detailed instructions for cryogenic recovery of compounds captured as whole air samples or thermally desorbed from sorbents are typically covered in standard methods for sample analysis and are beyond the scope of this guide.
1.4 Both thermal and solvent desorption techniques for sample recovery are discussed.
1.5 Organic compounds are classified on the basis of vapor pressure as very volatile, volatile, semivolatile and nonvolatile. Physical characteristics of many volatile organic compounds (VOCs) are provided to aid in selection of sampling techniques for VOC measurement. Semivolatile and nonvolatile organic compounds are defined in the guide to help guide users avoid misidentifying compounds that are not covered in this guide.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information.
´1
Designation:D6345–98 (Reapproved 2004)
Standard Guide for
Selection of Methods for Active, Integrative Sampling of
Volatile Organic Compounds in Air
This standard is issued under the fixed designation D6345; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
´ NOTE—Editorial changes were made to the title of Practice D 6196 in 2.1 and to Reference number (2) in April 2004.
1. Scope 2. Referenced Documents
1.1 This guide provides assistance in the selection of active 2.1 ASTM Standards:
integrative sampling methods, in which the volatile organic D1356 Terminology Relating to Sampling and Analysis of
analytesarecollectedfromairoveraperiodoftimebydrawing Atmospheres
the air into the sampling device, with subsequent recovery for D1357 Practice for Planning the Sampling of the Ambient
analysis. Where available, specific ASTM test methods and Atmosphere
practices are referenced. D3686 Practice for Sampling Atmospheres to Collect Or-
1.2 Guidance is provided for the selection of active sam- ganic Compound Vapors (Activated Charcoal Tube Ad-
pling methods based either on collection of an untreated air sorption Method)
sample(wholeairsamples)orselectivesamplingusingsorbent D3687 Practice for Analysis of Organic Compound Vapors
concentration techniques that selectively concentrate compo- Collected by the Activated Charcoal Tube Adsorption
nents in air. Advantages and disadvantages of specific collec- Method
tion vehicles are presented. D5197 Test Method for Determination of Formaldehyde
1.3 This guide does not cover the use of cryogenically and Other Carbonyl Compounds in Air (Active Sampler
cooled field sampling devices used in some automated analysis Methodology)
systems. Detailed instructions for cryogenic recovery of com- D5466 Test Method for Determination of Volatile Organic
pounds captured as whole air samples or thermally desorbed Chemicals in Atmospheres (Canister Sampling Methodol-
from sorbents are typically covered in standard methods for ogy)
sample analysis and are beyond the scope of this guide. D5953M Test Method for Determination of Non-Methane
1.4 Both thermal and solvent desorption techniques for Organic Compounds (NMOC) in Ambient Air Using
sample recovery are discussed. Cryogenic Preconcentration and Direct Flame Ionization
1.5 Organic compounds are classified on the basis of vapor Detection Method [Metric]
pressure as very volatile, volatile, semivolatile and nonvolatile. D6196 Practice for Selection of Sorbents, Sampling, and
Physical characteristics of many volatile organic compounds Thermal Desorption Analysis Procedures for Volatile Or-
(VOCs)areprovidedtoaidinselectionofsamplingtechniques ganic Compounds in Air
for VOC measurement. Semivolatile and nonvolatile organic
3. Terminology
compounds are defined in the guide to help guide users avoid
3.1 Definitions—For definitions of terms used in this guide
misidentifying compounds that are not covered in this guide.
1.6 This standard does not purport to address all of the refer to Terminology D1356.
3.2 Definitions of Terms Specific to This Standard:
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro- 3.2.1 cryofocus—the process of concentrating compounds
from an air sample for subsequent analysis by collection on a
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use. trap cooled with a cryogen to very low temperatures (for
example, -186°C).
This guide is under the jurisdiction ofASTM Committee D22 on Sampling and
Analysis of Atmospheres and is the direct responsibility of Subcommittee D22.05
on Indoor Air. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved April 1, 2004. Published June 2004. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
approved in 1998. Last previous edition approved in 1998 as D6345 - 98. DOI:
10.1520/D6345-98R04E01. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
´1
D6345–98 (2004)
3.2.1.1 Discussion—Cryogenic traps used for cryofocusing and experiences and incorporates them into condensed guide-
are typically U-shaped stainless steel tubes filled with glass lines. This guide provides a common basis for selecting
beads or other inert material.An example of such a cryofocus- methods for VOC measurement as well a discussion of the
ing trap is given in Test Method D5953M. Compounds are limitations of typical methods.
typically released from cryogenic traps into the analytical 4.2 This guide should be used during the planning stages of
system by rapid heating to elevated temperatures. Sorbent- an air monitoring program along with other applicable guides
filled tubes cooled to sub-ambient temperatures (for example, and practices (for example, D1357) to select ASTM or other
-30°C) have also been used for this purpose. appropriate methods.
3.2.2 very volatile organic compounds (VVOCs)—Low mo-
5. Characteristics of Organic Compounds
lecularweightorganiccompoundsthatpossessvaporpressures
greater than 15 kPa at 25°C and boiling points typically below 5.1 Physical and chemical characteristics of VOCs are
availablefromnumerousreferences(1,2,3,4). Theproperties
30°C.
of the VOCs listed under the Clean Air Act of 1990 (5) are
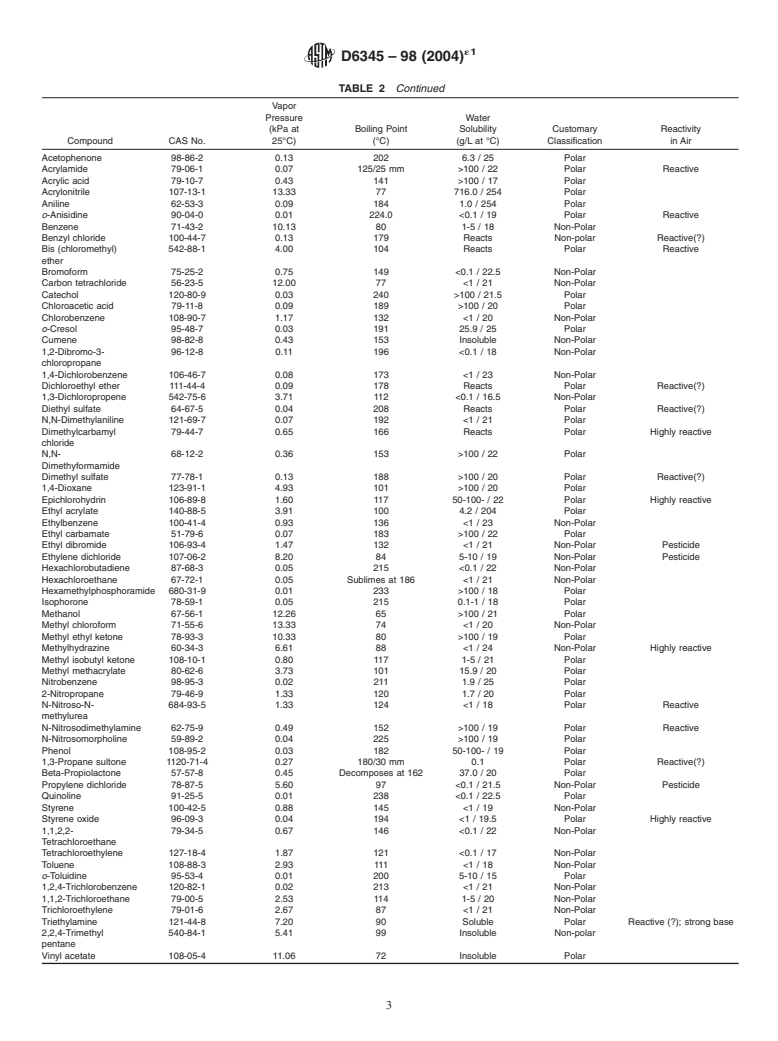
4. Significance and Use presented in Table 1 and Table 2.
4.1 This guide provides a broad perspective on techniques
thatcanbeusedbyenvironmentalmanagersforselectingVOC
air monitoring methods. It summarizes various methods for
The boldface numbers in parentheses refer to the list of references at the end of
measurement of VOC in air derived from a variety of sources this standard.
A,B
TABLE 1 Properties of Clean Air Act Very Volatile Organic HAPs
Vapor Boiling Water
Pressure Point Solubility (g/L Customary Reactivity in
Compound CAS No. (kPa at 25°C) (°C) at °C) Classification Air
Acetaldehyde 75-07-0 127 21 33.0 / 25 Polar
Acrolein 107-02-8 29 53 >100 /21 Polar Reactive
Allyl chloride 107-05-1 45 45 19.5 / 20 Non-Polar
1.3-Butadiene 106-99-0 267 -5 Insoluble Non-Polar Reactive (?)
Carbon disulfide 75-15-0 35 47 <1 / 20 Non-polar
Carbonyl sulfide 463-58-1 493 -50 >100 / 20 Polar
Chloroform 67-66-3 21 61 0.85 / 20-24 Non-Polar
Chloromethly methyl 107-30-2 30 59 Reacts Polar Reactive
ether
Chloroprene 126-99-8 30 59 Slightly soluble Non-Polar
Diazomethane 334-88-3 373 -23 Reacts Polar Highly reactive
1,1-Dimethylhydrazine 57-14-7 21 63 Reacts Non-Polar Reactive (?)
1,2-Epoxybutane 106-88-7 22 63 >100 / 17 Polar Reactive
Ethyl chloride 75-00-3 133 13 >100 / 20 Non-Polar
Ethyleneimine 151-56-4 21 56 Miscible Polar Reactive (?)
Ethylene oxide 75-21-8 147 11 Miscible Polar Reactive
Ethylidene dichloride 75-34-3 31 57 <1 / 20 Non-Polar
Formaldehyde 50-00-0 360 -20 >100 / 20.5 Polar
Hexane 110-54-3 16 69 <1 / 16.5 Non-Polar
Methyl bromide 74-83-9 240 4 Slightly soluble Non-Polar Pesticide
Methyl chloride 74-87-3 507 -24 Slightly soluble Non-Polar
Methyl iodide 74-88-4 53 42 10-50 / 18 Non-Polar
Methyl isocyanate 624-83-9 46 60 Reacts Polar Highly reactive
Methyl tert-butyl ether 1634-04-4 33 55 Soluble Polar
Methylene chloride 75-09-2 47 40 10-50 / 21 Non-Polar
Phosgene 75-44-5 160 8 Slightly soluble Polar Reactive (?)
Propionaldehyde 123-38-6 31 49 50-100 / 18 Polar Reactive
Propylene oxide 75-56-9 59 34 400 / 20 Polar Reactive
1,2-Propyleneimine 75-55-8 15 66 >100 / 19 Polar Highly reactive (?)
Vinyl bromide 593-60-2 147 16 Insoluble Non-Polar
Vinyl chloride 75-01-4 427 -14 Slightly soluble Non-Polar
Vinylidene chloride 75-35-4 67 32 5-10 / 21 Non-Polar
A
Compounds with vapor pressures > 15 kPa.
B
Data taken from Ref. (3).
A,B
TABLE 2 Properties of Clean Air Act Volatile Organic HAP
Vapor
Pressure Water
(kPa at Boiling Point Solubility Customary Reactivity
Compound CAS No. 25°C) (°C) (g/L at °C) Classification in Air
Acetonitrile 75-05-8 9.86 82 >100 / 22 Polar
´1
D6345–98 (2004)
TABLE 2 Continued
Vapor
Pressure Water
(kPa at Boiling Point Solubility Customary Reactivity
Compound CAS No. 25°C) (°C) (g/L at °C) Classification in Air
Acetophenone 98-86-2 0.13 202 6.3 / 25 Polar
Acrylamide 79-06-1 0.07 125/25 mm >100 / 22 Polar Reactive
Acrylic acid 79-10-7 0.43 141 >100 / 17 Polar
Acrylonitrile 107-13-1 13.33 77 716.0 / 254 Polar
Aniline 62-53-3 0.09 184 1.0 / 254 Polar
o-Anisidine 90-04-0 0.01 224.0 <0.1 / 19 Polar Reactive
Benzene 71-43-2 10.13 80 1-5 / 18 Non-Polar
Benzyl chloride 100-44-7 0.13 179 Reacts Non-polar Reactive(?)
Bis (chloromethyl) 542-88-1 4.00 104 Reacts Polar Reactive
ether
Bromoform 75-25-2 0.75 149 <0.1 / 22.5 Non-Polar
Carbon tetrachloride 56-23-5 12.00 77 <1 / 21 Non-Polar
Catechol 120-80-9 0.03 240 >100 / 21.5 Polar
Chloroacetic acid 79-11-8 0.09 189 >100 / 20 Polar
Chlorobenzene 108-90-7 1.17 132 <1 / 20 Non-Polar
o-Cresol 95-48-7 0.03 191 25.9 / 25 Polar
Cumene 98-82-8 0.43 153 Insoluble Non-Polar
1,2-Dibromo-3- 96-12-8 0.11 196 <0.1 / 18 Non-Polar
chloropropane
1,4-Dichlorobenzene 106-46-7 0.08 173 <1 / 23 Non-Polar
Dichloroethyl ether 111-44-4 0.09 178 Reacts Polar Reactive(?)
1,3-Dichloropropene 542-75-6 3.71 112 <0.1 / 16.5 Non-Polar
Diethyl sulfate 64-67-5 0.04 208 Reacts Polar Reactive(?)
N,N-Dimethylaniline 121-69-7 0.07 192 <1 / 21 Polar
Dimethylcarbamyl 79-44-7 0.65 166 Reacts Polar Highly reactive
chloride
N,N- 68-12-2 0.36 153 >100 / 22 Polar
Dimethyformamide
Dimethyl sulfate 77-78-1 0.13 188 >100 / 20 Polar Reactive(?)
1,4-Dioxane 123-91-1 4.93 101 >100 / 20 Polar
Epichlorohydrin 106-89-8 1.60 117 50-100- / 22 Polar Highly reactive
Ethyl acrylate 140-88-5 3.91 100 4.2 / 204 Polar
Ethylbenzene 100-41-4 0.93 136 <1 / 23 Non-Polar
Ethyl carbamate 51-79-6 0.07 183 >100 / 22 Polar
Ethyl dibromide 106-93-4 1.47 132 <1 / 21 Non-Polar Pesticide
Ethylene dichloride 107-06-2 8.20 84 5-10 / 19 Non-Polar Pesticide
Hexachlorobutadiene 87-68-3 0.05 215 <0.1 / 22 Non-Polar
Hexachloroethane 67-72-1 0.05 Sublimes at 186 <1 / 21 Non-Polar
Hexamethylphosphoramide 680-31-9 0.01 233 >100 / 18 Polar
Isophorone 78-59-1 0.05 215 0.1-1 / 18 Polar
Methanol 67-56-1 12.26 65 >100 / 21 Polar
Methyl chloroform 71-55-6 13.33 74 <1 / 20 Non-Polar
Methyl ethyl ketone 78-93-3 10.33 80 >100 / 19 Polar
Methylhydrazine 60-34-3 6.61 88 <1 / 24 Non-Polar Highly reactive
Methyl isobutyl ketone 108-10-1 0.80 117 1-5 / 21 Polar
Methyl methacrylate 80-62-6 3.73 101 15.9 / 20 Polar
Nitrobenzene 98-95-3 0.02 211 1.9 / 25 Polar
2-Nitropropane 79-46-9 1.33 120 1.7 / 20 Polar
N-Nitroso-N- 684-93-5 1.33 124 <1 / 18 Polar Reactive
methylurea
N-Nitrosodimethylamine 62-75-9 0.49 152 >100 / 19 Polar Reactive
N-Nitrosomorpholine 59-89-2 0.04 225 >100 / 19 Polar
Phenol 108-95-2 0.03 182 50-100- / 19 Polar
1,3-Propane sultone 1120-71-4 0.27 180/30 mm 0.1 Polar Reactive(?)
Beta-Propiolactone 57-57-8 0.45 Decomposes at 162 37.0 / 20 Polar
Propylene dichloride 78-87-5 5.60 97 <0.1 / 21.5 Non-Polar Pesticide
Quinoline 91-25-5 0.01 238 <0.1 / 22.5 Polar
Styrene 100-42-5 0.88 145 <1 / 19 Non-Polar
Styrene oxide 96-09-3 0.04 194 <1 / 19.5 Polar Highly reactive
1,1,2,2- 79-34-5 0.67 146 <0.1 / 22 Non-Polar
Tetrachloroethane
Tetrachloroethylene 127-18-4 1.87 121 <0.1 / 17 Non-Polar
Toluene 108-88-3 2.93 111 <1 / 18 Non-Polar
o-Toluidine 95-53-4 0.01 200 5-10 / 15 Polar
1,2,4-Trichlorobenzene 120-82-1 0.02 213 <1 / 21 Non-Polar
1,1,2-Trichloroethane 79-00-5 2.53 114 1-5 / 20 Non-Polar
Trichloroethylene 79-01-6 2.67 87 <1 / 21 Non-Polar
Triethylamine 121-44-8 7.20 90 Soluble Polar Reactive (?); strong base
2,2,4-Trimethyl 540-84-1 5.41 99 Insoluble Non-polar
pentane
Vinyl acetate 108-05-4 11.06 72 Insoluble Polar
´1
D6345–98 (2004)
TABLE 2 Continued
Vapor
Pressure Water
(kPa at Boiling Point Solubility Customary Reactivity
Compound CAS No. 25°C) (°C) (g/L at °C) Classification in Air
o-Xylene 95-47-6 0.67 144 Insoluble Non-Polar
m-Xylene 108-38-3 0.80 139 Insoluble Non-Polar
p-Xylene 106-42-3 0.87 138 Insoluble Non-Polar
A 2
Compounds with vapor pressures between 10 and 15 kPa.
B
Data taken from Ref. (4).
5.2 Organic compounds can be divided into four groups volume (ppbv). The conversion between these reporting units
based on volatility (1). is shown in Eq 1 and requires the molecular weight and the
5.2.1 VOCs with vapor pressures above 15 kPa at 25°C standard molar volume at standard temperature (273.15 K,
(boilingpointstypicallybelow30°C)aresometimesreferredto 0°C) and pressure (101.3 kPa, 760 mm Hg):
as very volatile organic compounds (VVOCs). At room tem-
C ppbv! 5 C µg/m ! 3 22.4/molecular weight (1)
~ ~
perature and atmospheric pressure, VVOCs are present in the
NOTE 2—Indoorsamplingisusuallyperformedattemperaturenear293
gas phase in air. Due to their high vapor pressures, VVOCs are
K (20°C). The standard molar volume at this temperature is 24.1 L/mol.
generally more difficult to collect and retain on sorbents than
other VOCs.
6. Selection of Sampling Methods for VOCs
5.2.2 Volatileorganiccompoundstypicallyhavevaporpres-
-2 6.1 The first criteria for selection of an appropriate method
suresabove10 kPaat25°C(typicalboilingpointsfromabout
for sampling are the physical and chemical characteristics of
30 to 180°C).VOCs with boiling points at the upper end of the
the compounds to be monitored. Once the analyte has been
range still have a significant vapor pressure at room tempera-
characterized as a volatile compound, the appropriate measure-
ture and atmospheric pressure. At room temperature and
ment method (sampling and analysis) is chosen. Sampling
atmospheric pressure VOCs are present in the gas phase in air.
methods can be active or passive.
5.2.3 Semivolatile organic compounds (SVOCs) typically
-2 -8 6.1.1 Active methods employ some means of setting and
have vapor pressures between 10 and 10 kPa at 25°C
controlling the air sampling rate (for example pump, syringe,
(typical boiling points from 180 to 350°C). SVOCs may be
or other vacuum source with a flow-controller).
present in both the vapor and particulate phases (1).
6.1.2 Passive/diffusive sampling methods have sampling
5.2.4 Nonvolatile organic compounds have vapor pressures
-8 rates that depend on the molecular diffusion rate, sampling
below 10 kPa at 25°C (boiling points typically above 300°C).
temperature, length and area of the diffusive
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.