ASTM E1981-98(2004)
(Guide)Standard Guide for Assessing the Thermal Stability of Materials by Methods of Accelerating Rate Calorimetry
Standard Guide for Assessing the Thermal Stability of Materials by Methods of Accelerating Rate Calorimetry
SIGNIFICANCE AND USE
The data from this guide seldom, if ever, directly simulate thermal and pressure events in the processing, storage, and shipping of chemicals. However, the data obtained from this guide may be used, with suitable precautions, to predict the thermal and pressure hazards associated with processing, storage, and shipping of a chemical or mixture of chemicals after appropriate scaling of the data. This has been addressed in the literature (1-9) but is beyond the scope of this guide.
This guide is suitable, under the proper conditions, for the investigation of the effects of catalyst, inhibitors, initiators, reaction atmospheres, materials of construction, or, if available, agitation (see 6.1.2).
Interpretation of the time-temperature or time-pressure data may be possible for relatively simple systems through the use of suitable temperature-dependent kinetic theories such as the Arrhenius and Absolute Reaction Rate theories (10-11).
SCOPE
1.1 This guide covers suggested procedures for the operation of a calorimetric device designed to obtain temperature and pressure data as a function of time for systems undergoing a physicochemical change under nearly adiabatic conditions.
1.2 This guide outlines the calculation of thermodynamic parameters from the time, temperature, and pressure data recorded by a calorimetric device.
1.3 The assessment outlined in this guide may be used over a pressure range from full vacuum to the rated pressure of the reaction container and pressure transducer. The temperature range of the calorimeter typically varies from ambient to 500&176C, but also may be user specified (see 6.6).
1.4 This statement does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific safety precautions are outlined in Section 7.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E1981 − 98(Reapproved 2004)
Standard Guide for
Assessing Thermal Stability of Materials by Methods of
Accelerating Rate Calorimetry
This standard is issued under the fixed designation E1981; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
INTRODUCTION
This guide is one of several standards being developed byASTM Committee E27 for determining
the physicochemical hazards of chemicals and chemical mixtures. This guide should be used in
conjunction with other test methods, as a complete assessment of the hazard potential of chemicals
must take into account a number of realistic factors not necessarily considered in this guide. The
expression hazard potential as used by this committee is defined as the degree of susceptibility of
material to ignition or release of energy under varying environmental conditions.
It is the intent of this guide to include any calorimetric device consistent with the principles of
adiabatic calorimetry. Device-specific information and specifications are located in appendices to the
guide. Any reference to specific devices in the guide are for purposes of illustration or clarity only.
1. Scope 2. Referenced Documents
1.1 This guide covers suggested procedures for the opera- 2.1 ASTM Standards:
tion of a calorimetric device designed to obtain temperature E476 Test Method for Thermal Instability of Confined Con-
and pressure data as a function of time for systems undergoing densed Phase Systems (Confinement Test) (Withdrawn
a physicochemical change under nearly adiabatic conditions. 2008)
E487 Test Method for Constant-Temperature Stability of
1.2 This guide outlines the calculation of thermodynamic
Chemical Materials
parameters from the time, temperature, and pressure data
E537 Test Method for The Thermal Stability of Chemicals
recorded by a calorimetric device.
by Differential Scanning Calorimetry
1.3 The assessment outlined in this guide may be used over
E680 Test Method for Drop Weight Impact Sensitivity of
a pressure range from full vacuum to the rated pressure of the
Solid-Phase Hazardous Materials
reaction container and pressure transducer. The temperature
E698 Test Method for Arrhenius Kinetic Constants for
range of the calorimeter typically varies from ambient to
Thermally Unstable Materials Using Differential Scan-
500°C, but also may be user specified (see 6.6).
ning Calorimetry and the Flynn/Wall/Ozawa Method
1.4 This statement does not purport to address all of the
E1231 Practice for Calculation of Hazard Potential Figures-
safety concerns, if any, associated with its use. It is the of-Merit for Thermally Unstable Materials
responsibility of the user of this standard to establish appro-
priate safety practices and to determine the applicability of 3. Terminology
regulatory limitations prior to use. Specific safety precautions
3.1 Definitions of Terms Specific to This Standard:
are outlined in Section 7.
1 2
This guide is under the jurisdiction of ASTM Committee E27 on Hazard For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Potential of Chemicals and is the direct responsibility of Subcommittee E27.02 on contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Thermal Stability and Condensed Phases. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved April 1, 2004. Published May 2004. Originally the ASTM website.
published in 1998. Last previous edition approved in 1998 as E1981 - 98. DOI: The last approved version of this historical standard is referenced on
10.1520/E1981-98R04. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E1981 − 98 (2004)
3.1.1 adiabatic calorimeter, n—an instrument capable of referenced from the time corresponding to the onset tempera-
making calorimetric measurements while maintaining a mini- ture, but may also be referenced from any time-temperature
mal heat loss or gain between the sample and its environment, point to the time at which the maximum self-heating or
which is verifiable by the capability to continuously measure pressure rate occurs. The experimentally observed TMR is
the temperature differential between the sample and its sur- normally divided by the thermal inertia factor (see 3.1.10)to
roundings. obtain a more conservative assessment ofTMR. (TMR divided
by the thermal inertia factor is often referred to as the
3.1.2 autocatalytic reaction, n—a chemical reaction in
“φ-corrected” TMR).
which a product or reaction intermediate functions as a
catalyst.
4. Summary of Guide
3.1.3 drift, n—a gradual unintended increase or decrease in
4.1 A sample is placed in a reaction container and posi-
the system (sample container and surroundings) temperature
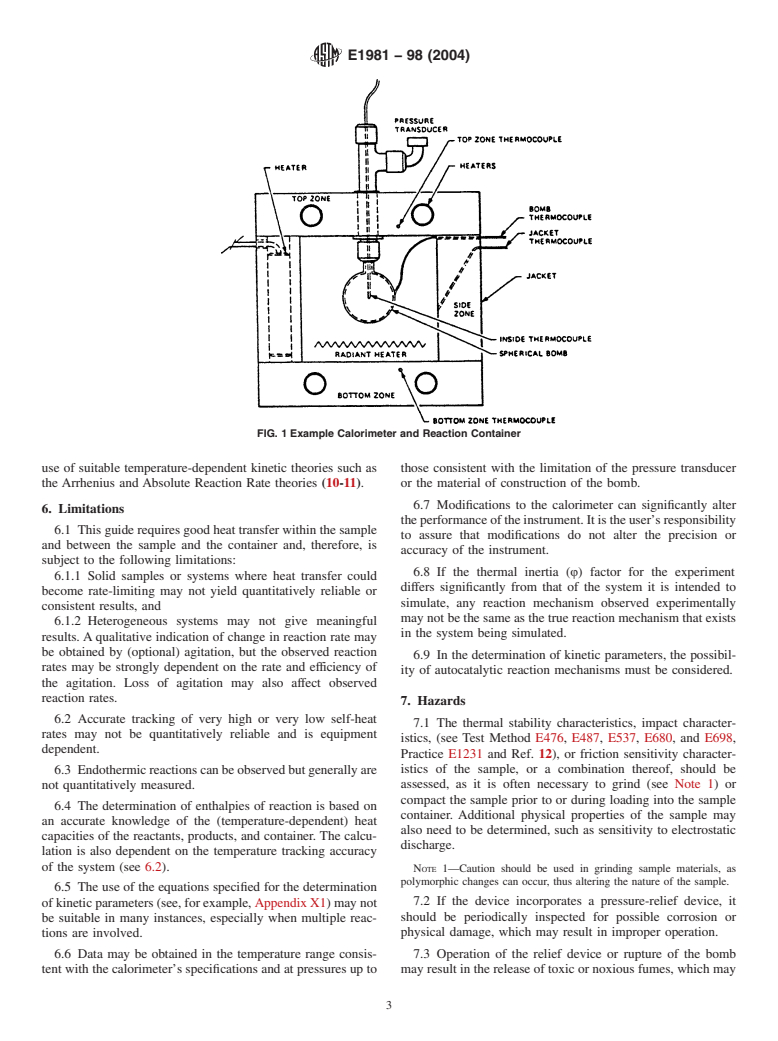
tioned in the calorimeter (see Fig. 1).
due to limitations in the system calibration, or to changes
4.2 The bomb is heated to a user-specified initial tempera-
which occur in the system after calibration.
ture and allowed to come to equilibrium, whereupon a search
3.1.4 final temperature (T ),n—the observed system
final
for evidence of an exothermic reaction is undertaken. An
temperature at the end of an exotherm, generally at the
exotherm is considered to have occurred when the user-
temperature where the self-heat rate of the reaction has
specified rate of temperature rise is first exceeded. If no
decreased below the operator-defined slope sensitivity thresh-
exotherm is detected, the system temperature is raised a
old.
specified increment and the system allowed to equilibrate
3.1.5 heat of reaction (∆H), n—the net calculated heat again. This heat-wait-search cycle is repeated until either an
(energy) liberated during an exothermic reaction. exotherm is detected or the upper temperature limit of the test
is reached. If an exotherm is detected, the surroundings are
3.1.6 ideal adiabatic temperature rise (∆T ), n—the tem-
ad
keptatthesametemperatureasthereactioncontainer,allowing
perature rise which would be observed in an exothermic
the system to be maintained without heat loss as the tempera-
reaction if all of the heat liberated were used to increase the
ture of the system increases due to the heat evolved during the
temperature of only the sample. It is conveniently calculated as
exotherm.
the product of the observed adiabatic temperature rise, ∆T ,
obs
and the thermal inertia factor, φ. 4.3 Time, temperature, and pressure data are recorded at
specified temperature intervals as a function of time. Addi-
3.1.7 observed adiabatic temperature rise (∆T ), n—the
obs
tional user-selected parameters may also be recorded or stored.
observed temperature rise in the system during an exotherm;
4.4 The recorded data are used to calculate the time rates of
mathematically, it is equal to the temperature difference be-
tween the final temperature and the onset temperature of an changes of pressure and temperature. These data may also be
exotherm. usedtocalculateatime-to-maximumrate(asdefinedin3.1.12)
and to obtain kinetic parameters (1-9) for simple, non-
3.1.8 onset temperature (T ),n—the observed system
start
autocatalytic exothermic reactions using the equations speci-
temperature at the start of an exotherm where the self-heating
fied in the vendors’ manual (subject to the limitations of 6.5).
rate first exceeds the operator-defined slope sensitivity thresh-
These data may also be adjusted for the sample- and container-
old, usually 0.02°C/min; the onset temperature is not a funda-
specific heats to calculate an adiabatic temperature rise and
mental property of a substance, but is apparatus-dependent,
heat of reaction.
based upon the inherent sensitivity of the calorimetric system.
5. Significance and Use
3.1.9 self-heating, adj—any exothermic process which in-
creases the temperature of the system by the self absorption of
5.1 The data from this guide seldom, if ever, directly
the liberated heat.
simulatethermalandpressureeventsintheprocessing,storage,
3.1.10 thermal inertia factor (φ), n— a correction factor and shipping of chemicals. However, the data obtained from
thisguidemaybeused,withsuitableprecautions,topredictthe
applied to time and temperature differences observed in exo-
thermic reactions in the system (sample and container) under thermal and pressure hazards associated with processing,
storage, and shipping of a chemical or mixture of chemicals
test, which accounts for the sensible heat absorbed by the
sample container that otherwise would lead to erroneously low afterappropriatescalingofthedata.Thishasbeenaddressedin
the literature (1-9) but is beyond the scope of this guide.
heats of reaction and adiabatic temperature rise, as well as to
erroneously high time to maximum rates (TMR’s) (see 3.1.12).
5.2 This guide is suitable, under the proper conditions, for
See also 10.1 for a mathematical formula definition of the
the investigation of the effects of catalyst, inhibitors, initiators,
thermal inertia factor.
reaction atmospheres, materials of construction, or, if avail-
able, agitation (see 6.1.2).
3.1.11 thermal runaway reaction, n—a chemical reaction in
which the heat generation rate in a system exceeds the heat
5.3 Interpretation of the time-temperature or time-pressure
removal rate of that system.
data may be possible for relatively simple systems through the
3.1.12 time-to-maximum rate (TMR), n—the amount of time
that is needed for a reaction to reach its maximum self-heating
The boldface numbers in parentheses refer to the list of references found at the
rate or pressure rate in a thermal runaway reaction, normally end of this practice.
E1981 − 98 (2004)
FIG. 1 Example Calorimeter and Reaction Container
use of suitable temperature-dependent kinetic theories such as those consistent with the limitation of the pressure transducer
the Arrhenius and Absolute Reaction Rate theories (10-11). or the material of construction of the bomb.
6.7 Modifications to the calorimeter can significantly alter
6. Limitations
theperformanceoftheinstrument.Itistheuser’sresponsibility
6.1 This guide requires good heat transfer within the sample
to assure that modifications do not alter the precision or
and between the sample and the container and, therefore, is
accuracy of the instrument.
subject to the following limitations:
6.8 If the thermal inertia (φ) factor for the experiment
6.1.1 Solid samples or systems where heat transfer could
differs significantly from that of the system it is intended to
become rate-limiting may not yield quantitatively reliable or
simulate, any reaction mechanism observed experimentally
consistent results, and
may not be the same as the true reaction mechanism that exists
6.1.2 Heterogeneous systems may not give meaningful
in the system being simulated.
results.Aqualitative indication of change in reaction rate may
be obtained by (optional) agitation, but the observed reaction
6.9 In the determination of kinetic parameters, the possibil-
rates may be strongly dependent on the rate and efficiency of
ity of autocatalytic reaction mechanisms must be considered.
the agitation. Loss of agitation may also affect observed
reaction rates.
7. Hazards
6.2 Accurate tracking of very high or very low self-heat
7.1 The thermal stability characteristics, impact character-
rates may not be quantitatively reliable and is equipment
istics, (see Test Method E476, E487, E537, E680, and E698,
dependent.
Practice E1231 and Ref. 12), or friction sensitivity character-
6.3 Endothermicreactionscanbeobservedbutgenerallyare istics of the sample, or a combination thereof, should be
assessed, as it is often necessary to grind (see Note 1)or
not quantitatively measured.
compact the sample prior to or during loading into the sample
6.4 The determination of enthalpies of reaction is based on
container. Additional physical properties of the sample may
an accurate knowledge of the (temperature-dependent) heat
also need to be determined, such as sensitivity to electrostatic
capacities of the reactants, products, and container. The calcu-
discharge.
lation is also dependent on the temperature tracking accuracy
of the system (see 6.2).
NOTE 1—Caution should be used in grinding sample materials, as
polymorphic changes can occur, thus altering the nature of the sample.
6.5 The use of the equations specified for the determination
7.2 If the device incorporates a pressure-relief device, it
ofkineticparameters(see,forexample,AppendixX1)maynot
should be periodically inspected for possible corrosion or
be suitable in many instances, especially when multiple reac-
physical damage, which may result in improper operation.
tions are involved.
6.6 Data may be obtained in the temperature range consis- 7.3 Operation of the relief device or rupture of the bomb
tent with the calorimeter’s specifications and at pressures up to may result in the release of toxic or noxious fumes, which may
E1981 − 98 (2004)
escape into the immediate operating area. The calorimeter, 8.3 Optional components include provision for stirring of
therefore, should be properly vented. the sample and automatic operation (data collection and
storage) of the apparatus after test initialization.
7.4 Whenventingthesamplecontainerattheendofthetest,
suitable precautions should be taken prior to lifting the top 8.4 The apparatus shall employ the principles of adiabatic
calorimetry to minimize heat loss from the reaction container
cover of the calorimeter in order to prevent exposure of the
operator to a potentially highly pressurized container capable to the surrounding environment.
of rupture without warning.
8.5 The calorimeter shall be adequately shielded and vented
7.5 Bombs and transducer lines may become plugged, in order to protect the operator from any sample container
rupture or detonation within the calorimeter and from any
preventing normal operation of any relief device or vent valve.
Therefore, exercise caution and use appropriate personal pro- resulting effluent. (see Section 7).
tective equipment and shielding devices prior to attempts to
9. Procedure
relieve the pressure. Depressurization and subsequent opening
of the sample container at the end of the test should be
9.1 Calibration:
performedinasafemanner,takingintoconsiderationpotential,
9.1.1 An instrument calibration should be carried out on a
unanticipated pressure releases or exposure to the operator, or
regular basis or whenever a major change has b
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.