ASTM E2160-04(2018)
(Test Method)Standard Test Method for Heat of Reaction of Thermally Reactive Materials by Differential Scanning Calorimetry
Standard Test Method for Heat of Reaction of Thermally Reactive Materials by Differential Scanning Calorimetry
SIGNIFICANCE AND USE
5.1 This test method is useful in determining the extrapolated onset temperature, the peak heat flow temperature and the heat of reaction of a material. Any onset temperature determined by this test method is not valid for use as the sole information used for determination of storage or processing conditions.
5.2 This test method is useful in determining the fraction of a reaction that has been completed in a sample prior to testing. This fraction of reaction that has been completed can be a measure of the degree of cure of a thermally reactive polymer or can be a measure of decomposition of a thermally reactive material upon aging.
5.3 The heat of reaction values may be used in Practice E1231 to determine hazard potential figures-of-merit Explosion Potential and Shock Sensitivity.
5.4 This test method may be used in research, process control, quality assurance, and specification acceptance.
SCOPE
1.1 This test method determines the exothermic heat of reaction of thermally reactive chemicals or chemical mixtures, using milligram specimen sizes, by differential scanning calorimetry. Such reactive materials may include thermally unstable or thermoset materials.
1.2 This test method also determines the extrapolated onset temperature and peak heat flow temperature for the exothermic reaction.
1.3 This test method may be performed on solids, liquids or slurries.
1.4 The applicable temperature range of this test method is 25 to 600°C.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 There is no ISO method equivalent to this standard.
1.7 This standard is related to Test Method E537 and to NAS 1613, but provides additional information.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.9 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E2160 − 04 (Reapproved 2018)
Standard Test Method for
Heat of Reaction of Thermally Reactive Materials by
Differential Scanning Calorimetry
This standard is issued under the fixed designation E2160; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 This test method determines the exothermic heat of
E473 Terminology Relating to Thermal Analysis and Rhe-
reaction of thermally reactive chemicals or chemical mixtures,
ology
using milligram specimen sizes, by differential scanning calo-
E537 Test Method for The Thermal Stability of Chemicals
rimetry. Such reactive materials may include thermally un-
by Differential Scanning Calorimetry
stable or thermoset materials.
E967 Test Method for Temperature Calibration of Differen-
1.2 This test method also determines the extrapolated onset tial Scanning Calorimeters and Differential Thermal Ana-
temperature and peak heat flow temperature for the exothermic lyzers
E968 Practice for Heat Flow Calibration of Differential
reaction.
Scanning Calorimeters
1.3 This test method may be performed on solids, liquids or
E1142 Terminology Relating to Thermophysical Properties
slurries.
E1231 Practice for Calculation of Hazard Potential Figures
of Merit for Thermally Unstable Materials
1.4 The applicable temperature range of this test method is
E1860 Test Method for Elapsed Time Calibration of Ther-
25 to 600°C.
mal Analyzers
1.5 The values stated in SI units are to be regarded as
2.2 Other Standard:
standard. No other units of measurement are included in this NAS 1613 Seal Element, Packing, Preformed, Ethylene
standard. Propylene Rubber
1.6 There is no ISO method equivalent to this standard.
3. Terminology
3.1 Specifictechnicaltermsusedinthisstandardaredefined
1.7 This standard is related to Test Method E537 and to
in Terminologies E473 and E1142.
NAS 1613, but provides additional information.
1.8 This standard does not purport to address all of the
4. Summary of Test Method
safety concerns, if any, associated with its use. It is the
4.1 A small (milligram) quantity of the reactive material is
responsibility of the user of this standard to establish appro-
heated at 10°C/min through a temperature region where a
priate safety, health, and environmental practices and deter-
chemical reaction takes place. The exothermic heat flow
mine the applicability of regulatory limitations prior to use.
produced by the reaction is recorded as a function of tempera-
1.9 This international standard was developed in accor- ture and time by a differential scanning calorimeter. Integration
dance with internationally recognized principles on standard- of the exothermic heat flow over time yields the heat of
reaction. If the heat flow is endothermic, then this test method
ization established in the Decision on Principles for the
is not to be used.
Development of International Standards, Guides and Recom-
mendations issued by the World Trade Organization Technical
4.2 The test method can be used to determine the fraction of
Barriers to Trade (TBT) Committee.
a reaction that has occurred in a partially reacted sample. The
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
ThistestmethodisunderthejurisdictionofASTMCommitteeE37onThermal contact ASTM Customer service at service@astm.org. For Annual Book of ASTM
Measurements and is the direct responsibility of Subcommittee E37.01 on Calo- Standards volume information, refer to the standard’s Document Summary page on
rimetry and Mass Loss. the ASTM website.
Current edition approved April 1, 2018. Published May 2018. Originally Available from National Aerospace Standard (NAS), Aerospace Industries
approved in 2001. Last previous edition approved in 2012 as E2160 – 04 (2012). Association (AIA), 1000 Wilson Blvd., Suite 1700, Arlington, VA 22209, http://
DOI: 10.1520/E2160-04R18. www.aia-aerospace.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2160 − 04 (2018)
heat of reaction is determined for a specimen that is known to 6.1.1.6 RecordingDevice, capable of recording and display-
be 100 % unreacted and is compared to the heat of reaction ing any portion (including signal noise) of the differential heat
determined for the partially reacted sample. Appropriate cal- flow on the ordinate as a function of temperature or time on the
culation yields the fraction of the latter sample that was abscissa.
unreacted.
6.2 Containers, (pans, crucibles, vials, etc. and lids) that are
4.3 Subtracting the reaction fraction remaining from unity inert to the specimen and reference materials and that are of
suitable structural shape and integrity to contain the specimen
(1) yields the fraction reacted. The fraction reacted may be
expressed as percent. If the sample tested is a thermoset resin, and reference in accordance with the specific requirements of
this test method.
the percent reacted is often called the percent of cure.
6.3 Balance, with a capacity of 100 mg or greater to weigh
4.4 The extrapolated onset temperature and peak heat flow
specimens and containers, or both, to a sensitivity of 61 µg.
temperature are determined for the exothermic heat flow
thermal curve from 4.1.
7. Safety Precautions
5. Significance and Use
7.1 The use of this test method for materials of unknown
potential hazards requires that precautions be taken during the
5.1 This test method is useful in determining the extrapo-
sample preparation and testing.
latedonsettemperature,thepeakheatflowtemperatureandthe
heat of reaction of a material. Any onset temperature deter- 7.2 Where particle size reduction by grinding is necessary,
mined by this test method is not valid for use as the sole
the user of this test method shall presume that the material is
information used for determination of storage or processing hazardous.
conditions.
7.3 Toxic or corrosive effluents, or both, may be released
5.2 This test method is useful in determining the fraction of when heating the test specimen and could be harmful to
a reaction that has been completed in a sample prior to testing. personnelortheapparatus.Useofanexhaustsystemtoremove
This fraction of reaction that has been completed can be a such effluents is recommended.
measure of the degree of cure of a thermally reactive polymer
or can be a measure of decomposition of a thermally reactive 8. Calibration
material upon aging.
8.1 Perform any calibration procedures recommended by
the apparatus manufacturer as described in the Operations
5.3 The heat of reaction values may be used in Practice
Manual.
E1231 to determine hazard potential figures-of-merit Explo-
sion Potential and Shock Sensitivity.
8.2 Calibrate the temperature signal to within 62°C using
Test Method E967.
5.4 This test method may be used in research, process
control, quality assurance, and specification acceptance.
8.3 Calibrate the heat flow signal to within 60.5 % using
Practice E968.
6. Apparatus
8.4 Calibrate the elapsed time signal, or ascertain its
6.1 Differential Scanning Calorimeter (DSC), capable of
accuracy, to within 60.5 % using Test Method E1860.
measuringandrecordingheatflowasafunctionoftemperature
and time. Such a DSC is composed of:
9. Procedure
6.1.1 Test Chamber, composed of:
9.1 Into a tared sample container, weigh to within 61µg, 1
6.1.1.1 Furnace(s),toprovideuniformcontrolledheatingof
to 2 mg of the test specimen. Record this mass as M in mg.
a specimen and reference to a constant temperature or at a
Close the sample. Weigh the sealed container to within 61µg
constant rate within the temperature range of 25 to 600°C.
and recorded this mass as N in mg.
6.1.1.2 Temperature Sensor, to provide an indication of the
NOTE 2—Because of the reactive nature of the materials examined by
specimen or furnace temperature to within 60.5°C.
this test method, small specimen sizes shall be used unless the approxi-
6.1.1.3 Differential Sensor, to detect a heat flow difference
mate reactivity of the test specimen is known. Other specimen sizes may
between the specimen and reference equivalent to 0.2 mW. be used but shall be reported. Make sure that the specimen is homogenous
and represents the sample.
6.1.1.4 Means of Sustaining a Test Chamber Environment,
NOTE 3—Some substances may have non-reactive components mixed
of inert (for example, nitrogen, helium or argon) or reactive
with the thermally reactive material. An example would be reinforcing
(for example, air) gas at a purge rate of 50 6 5 mL/min.
fibers mixed with a thermally-curing polymer. A specification of the
fraction of inert material in the mixture may accompany these materials.
NOTE 1—Typically, at least 99 % pure nitrogen, helium or argon is
The user should be aware that such specifications involve tolerances so
employed when oxidation in air is a concern. Unless effects of moisture
that the actual fraction of inert material may vary within these tolerances
are to be studied, use of dry purge gas is recommended.
from lot to lot. In such cases, the actual fraction of inert material must be
taken into account.
6.1.1.5 Temperature Controller, capable of executing a spe-
NOTE 4—For highly reactive materials, the selection of sample con-
cific temperature program by operating the furnace(s) between
tainers can be particularly important. The material from which the
selected temperature limits (ambient temperature to 600°C) at
containerisconstructedmaycatalyzethereactionorreactwiththesample
a heating rate between 2 and 20°C/min constant to within
material. Sealed containers may cause an autocatalytic effect or possibly
60.1°C/min. a pressure effect. In open containers loss of material, and thereby loss of
E2160 − 04 (2018)
heat,couldbeanissue.Excessivepressurizationofasamplecontainercan integration must be performed with the abscissa presented as a time
be avoided by using vented containers, however, vented or unsealed (seconds) coordinate.
containers may cause the measured heat of reaction to be much smaller
NOTE 8—The amount of material should be chosen such that the
than the true value. See 12.4 for an example of such an effect.
maximum heat flow is less than 8 mW. This requirement reduces the
potentialofobtainingadiabaticheatingofthesample.Adiabaticheatingof
9.2 Heat the test specimen at a controlled rate of 10 6
the sample results in “leaning” peaks, an example of which is shown in
0.1°C/min from ambient until the thermal curve returns to
Fig. 2 (adapted from Figure 11 of Jones (1996)). . For highly energetic
bas
...
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E2160 − 04 (Reapproved 2018)
Standard Test Method for
Heat of Reaction of Thermally Reactive Materials by
Differential Scanning Calorimetry
This standard is issued under the fixed designation E2160; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 This test method determines the exothermic heat of
E473 Terminology Relating to Thermal Analysis and Rhe-
reaction of thermally reactive chemicals or chemical mixtures,
ology
using milligram specimen sizes, by differential scanning calo-
E537 Test Method for The Thermal Stability of Chemicals
rimetry. Such reactive materials may include thermally un-
by Differential Scanning Calorimetry
stable or thermoset materials.
E967 Test Method for Temperature Calibration of Differen-
1.2 This test method also determines the extrapolated onset tial Scanning Calorimeters and Differential Thermal Ana-
lyzers
temperature and peak heat flow temperature for the exothermic
E968 Practice for Heat Flow Calibration of Differential
reaction.
Scanning Calorimeters
1.3 This test method may be performed on solids, liquids or
E1142 Terminology Relating to Thermophysical Properties
slurries.
E1231 Practice for Calculation of Hazard Potential Figures
of Merit for Thermally Unstable Materials
1.4 The applicable temperature range of this test method is
E1860 Test Method for Elapsed Time Calibration of Ther-
25 to 600°C.
mal Analyzers
1.5 The values stated in SI units are to be regarded as
2.2 Other Standard:
standard. No other units of measurement are included in this
NAS 1613 Seal Element, Packing, Preformed, Ethylene
standard. Propylene Rubber
1.6 There is no ISO method equivalent to this standard.
3. Terminology
3.1 Specific technical terms used in this standard are defined
1.7 This standard is related to Test Method E537 and to
in Terminologies E473 and E1142.
NAS 1613, but provides additional information.
1.8 This standard does not purport to address all of the
4. Summary of Test Method
safety concerns, if any, associated with its use. It is the
4.1 A small (milligram) quantity of the reactive material is
responsibility of the user of this standard to establish appro-
heated at 10°C/min through a temperature region where a
priate safety, health, and environmental practices and deter-
chemical reaction takes place. The exothermic heat flow
mine the applicability of regulatory limitations prior to use.
produced by the reaction is recorded as a function of tempera-
1.9 This international standard was developed in accor-
ture and time by a differential scanning calorimeter. Integration
dance with internationally recognized principles on standard- of the exothermic heat flow over time yields the heat of
ization established in the Decision on Principles for the reaction. If the heat flow is endothermic, then this test method
is not to be used.
Development of International Standards, Guides and Recom-
mendations issued by the World Trade Organization Technical
4.2 The test method can be used to determine the fraction of
Barriers to Trade (TBT) Committee.
a reaction that has occurred in a partially reacted sample. The
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
This test method is under the jurisdiction of ASTM Committee E37 on Thermal contact ASTM Customer service at service@astm.org. For Annual Book of ASTM
Measurements and is the direct responsibility of Subcommittee E37.01 on Calo- Standards volume information, refer to the standard’s Document Summary page on
rimetry and Mass Loss. the ASTM website.
Current edition approved April 1, 2018. Published May 2018. Originally Available from National Aerospace Standard (NAS), Aerospace Industries
approved in 2001. Last previous edition approved in 2012 as E2160 – 04 (2012). Association (AIA), 1000 Wilson Blvd., Suite 1700, Arlington, VA 22209, http://
DOI: 10.1520/E2160-04R18. www.aia-aerospace.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2160 − 04 (2018)
heat of reaction is determined for a specimen that is known to 6.1.1.6 Recording Device, capable of recording and display-
be 100 % unreacted and is compared to the heat of reaction ing any portion (including signal noise) of the differential heat
determined for the partially reacted sample. Appropriate cal- flow on the ordinate as a function of temperature or time on the
culation yields the fraction of the latter sample that was abscissa.
unreacted.
6.2 Containers, (pans, crucibles, vials, etc. and lids) that are
inert to the specimen and reference materials and that are of
4.3 Subtracting the reaction fraction remaining from unity
(1) yields the fraction reacted. The fraction reacted may be suitable structural shape and integrity to contain the specimen
and reference in accordance with the specific requirements of
expressed as percent. If the sample tested is a thermoset resin,
the percent reacted is often called the percent of cure. this test method.
6.3 Balance, with a capacity of 100 mg or greater to weigh
4.4 The extrapolated onset temperature and peak heat flow
specimens and containers, or both, to a sensitivity of 61 µg.
temperature are determined for the exothermic heat flow
thermal curve from 4.1.
7. Safety Precautions
5. Significance and Use
7.1 The use of this test method for materials of unknown
potential hazards requires that precautions be taken during the
5.1 This test method is useful in determining the extrapo-
sample preparation and testing.
lated onset temperature, the peak heat flow temperature and the
heat of reaction of a material. Any onset temperature deter-
7.2 Where particle size reduction by grinding is necessary,
mined by this test method is not valid for use as the sole the user of this test method shall presume that the material is
information used for determination of storage or processing
hazardous.
conditions.
7.3 Toxic or corrosive effluents, or both, may be released
5.2 This test method is useful in determining the fraction of when heating the test specimen and could be harmful to
a reaction that has been completed in a sample prior to testing. personnel or the apparatus. Use of an exhaust system to remove
This fraction of reaction that has been completed can be a such effluents is recommended.
measure of the degree of cure of a thermally reactive polymer
8. Calibration
or can be a measure of decomposition of a thermally reactive
material upon aging.
8.1 Perform any calibration procedures recommended by
the apparatus manufacturer as described in the Operations
5.3 The heat of reaction values may be used in Practice
Manual.
E1231 to determine hazard potential figures-of-merit Explo-
sion Potential and Shock Sensitivity.
8.2 Calibrate the temperature signal to within 62°C using
Test Method E967.
5.4 This test method may be used in research, process
control, quality assurance, and specification acceptance.
8.3 Calibrate the heat flow signal to within 60.5 % using
Practice E968.
6. Apparatus
8.4 Calibrate the elapsed time signal, or ascertain its
6.1 Differential Scanning Calorimeter (DSC), capable of
accuracy, to within 60.5 % using Test Method E1860.
measuring and recording heat flow as a function of temperature
and time. Such a DSC is composed of: 9. Procedure
6.1.1 Test Chamber, composed of:
9.1 Into a tared sample container, weigh to within 61µg, 1
6.1.1.1 Furnace(s), to provide uniform controlled heating of
to 2 mg of the test specimen. Record this mass as M in mg.
a specimen and reference to a constant temperature or at a
Close the sample. Weigh the sealed container to within 61 µg
constant rate within the temperature range of 25 to 600°C.
and recorded this mass as N in mg.
6.1.1.2 Temperature Sensor, to provide an indication of the
NOTE 2—Because of the reactive nature of the materials examined by
specimen or furnace temperature to within 60.5°C.
this test method, small specimen sizes shall be used unless the approxi-
6.1.1.3 Differential Sensor, to detect a heat flow difference
mate reactivity of the test specimen is known. Other specimen sizes may
between the specimen and reference equivalent to 0.2 mW. be used but shall be reported. Make sure that the specimen is homogenous
and represents the sample.
6.1.1.4 Means of Sustaining a Test Chamber Environment,
NOTE 3—Some substances may have non-reactive components mixed
of inert (for example, nitrogen, helium or argon) or reactive
with the thermally reactive material. An example would be reinforcing
(for example, air) gas at a purge rate of 50 6 5 mL/min.
fibers mixed with a thermally-curing polymer. A specification of the
fraction of inert material in the mixture may accompany these materials.
NOTE 1—Typically, at least 99 % pure nitrogen, helium or argon is
The user should be aware that such specifications involve tolerances so
employed when oxidation in air is a concern. Unless effects of moisture
that the actual fraction of inert material may vary within these tolerances
are to be studied, use of dry purge gas is recommended.
from lot to lot. In such cases, the actual fraction of inert material must be
taken into account.
6.1.1.5 Temperature Controller, capable of executing a spe-
NOTE 4—For highly reactive materials, the selection of sample con-
cific temperature program by operating the furnace(s) between
tainers can be particularly important. The material from which the
selected temperature limits (ambient temperature to 600°C) at
container is constructed may catalyze the reaction or react with the sample
a heating rate between 2 and 20°C/min constant to within
material. Sealed containers may cause an autocatalytic effect or possibly
60.1°C/min. a pressure effect. In open containers loss of material, and thereby loss of
E2160 − 04 (2018)
heat, could be an issue. Excessive pressurization of a sample container can integration must be performed with the abscissa presented as a time
be avoided by using vented containers, however, vented or unsealed
(seconds) coordinate.
containers may cause the measured heat of reaction to be much smaller
NOTE 8—The amount of material should be chosen such that the
than the true value. See 12.4 for an example of such an effect.
maximum heat flow is less than 8 mW. This requirement reduces the
potential of obtaining adiabatic heating of the sample. Adiabatic heating of
9.2 Heat the test specimen at a controlled rate of 10 6
the sample results in “leaning” peaks, an example of which is shown in
0.1°C/min from ambient until the thermal curve returns to
Fig. 2 (adapted from Figure 11 of Jones (1996)). . For highly energetic
baseline following the exothermic event. If the upper limit of
materials, it might be impossible to satisfy simultaneously the direction of
temperature for this test method, 600°C, is reached before the
9.1 (using 1 to 2 mg of the test specimen) and the condition of this note
thermal curve returns to baseline, then this test method is not (maximum heat flow less than 8 mW). If heat flow is larger than 8 mW and
the peak is not “leaning”, it should not be necessary to reduce sample
applicable.
mass. Or, in other words, when both directions cannot be met
NOTE 5—Other heating rates may be used but shall be reported.
simultaneously, sample mass need be reduced only if the observe peak
leans.
9.3 Cool the test specimen to ambient temperature upon
completion of the experiment.
9.7 Construct a tangent to the leading edge of the exother-
mic peak at the point of maximum rate of change and
9.4 Reweigh the sample container. Compare this mass of the
extrapolate that tangent to the baseline constructed in 9.5.
sealed sample container weight with N determined in 9.1.
Record the intersection of the tangent with the baseline as the
Report any specimen weight loss observed.
onset temperature (To).
9.5 Construct a line connecting the baseline before the
exothermic reaction to that after the reaction (see Fig. 1).
NOTE 9—In some cases, reactions may have induction periods or other
effects that are manifested as exothermic deviations from the established
NOTE 6—For highly energetic reactions, a significant change may occur
baseline well before the onset temperature obtained by 9.7. Because of the
in the baseline prior to and following the exothermic reaction, due to a
importance of these effects for highly reactive materials, an additional
significant change in the heat capacity of the reacted material in the
onset temperature, the temperature of first deviation (Tf), is to be reported
sample container. Such an instance might be handled by the construction
also. The temperature of first deviation is the temperature for which the
of a baseline that is not a straight line. If a nonlinear baseline (for example,
thermal curve first deviates from the established baseline. The temperature
a sigmoidal baseline) is used it should be stated in the report and an
of first deviation is to be noted in the report.
example of the constructed baseline and the thermal curve should be
NOTE 10—Peak temperatures from two different determinations are
included also.
comparable only if the same conditions were used for both measurements,
9.6 Integrate the area, as a function of time, bounded by the
for example, sample mass and vent diameter.
thermal curve and the baseline constructed in 9.5. Record this
9.8 Record the temperature at the maximum deflection from
area as the heat of the reaction (A) in mJ.
the baseline constructed in 9.5 as the peak temperature (Tp).
NOTE 7—The area bounded by the thermal curve and the constructed
baseline gives the heat of the reaction. Instrument software is most often
used to integrate this area. Although such software packages display
thermal curves as in Fig. 1, they calculate the bound area on a basis of
Jones, D.E.G., and Augsten R.A., “Evaluation of Systems for Use in DSC
time. If older instruments without these software packages are used, or if
Measurements on Energetic Materials,” Thermochimica Acta, Vol 286, 1996, pp.
manual checks are performed on newer instruments, then the manual 355–373.
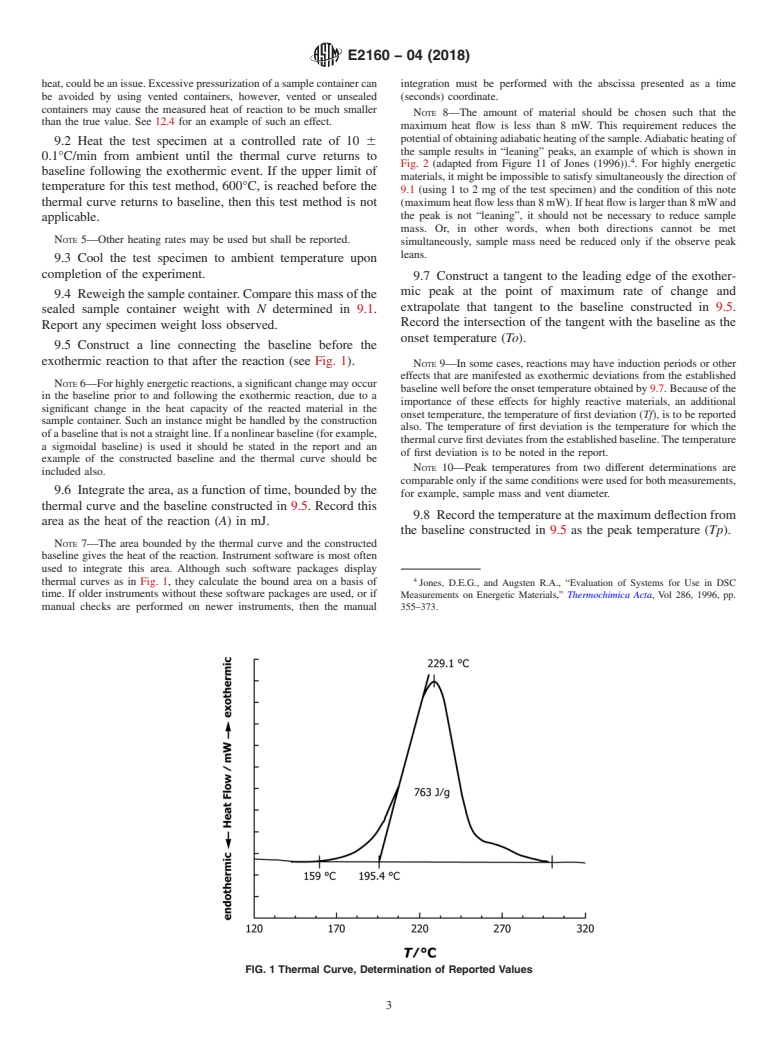
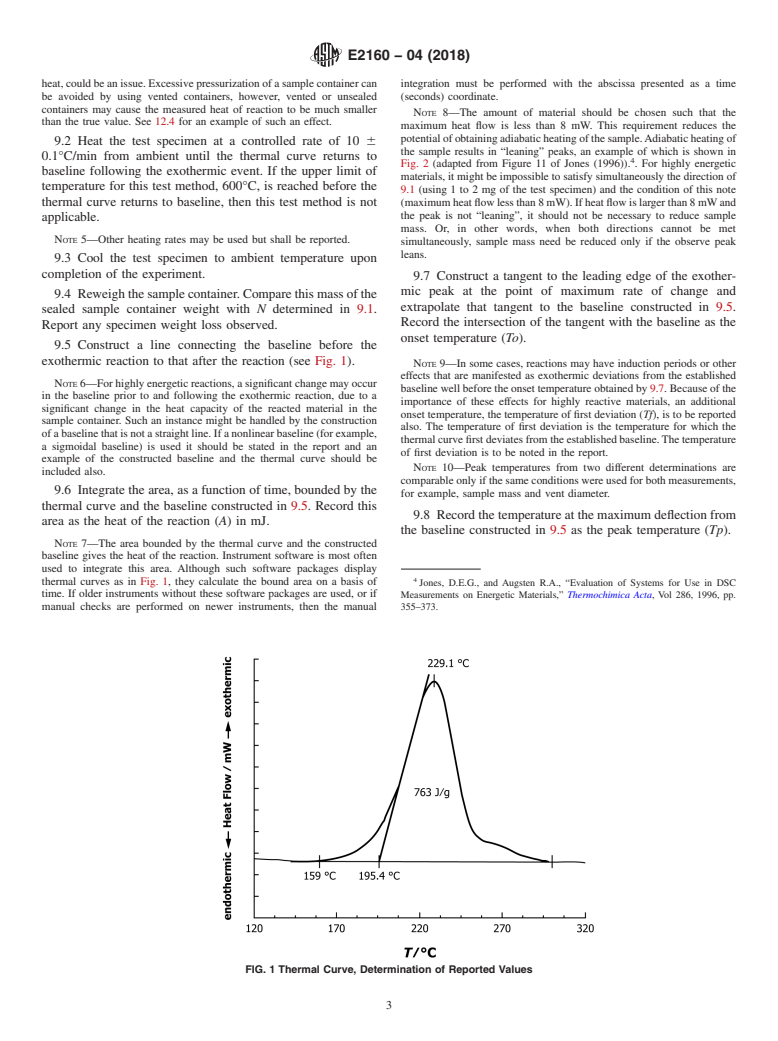
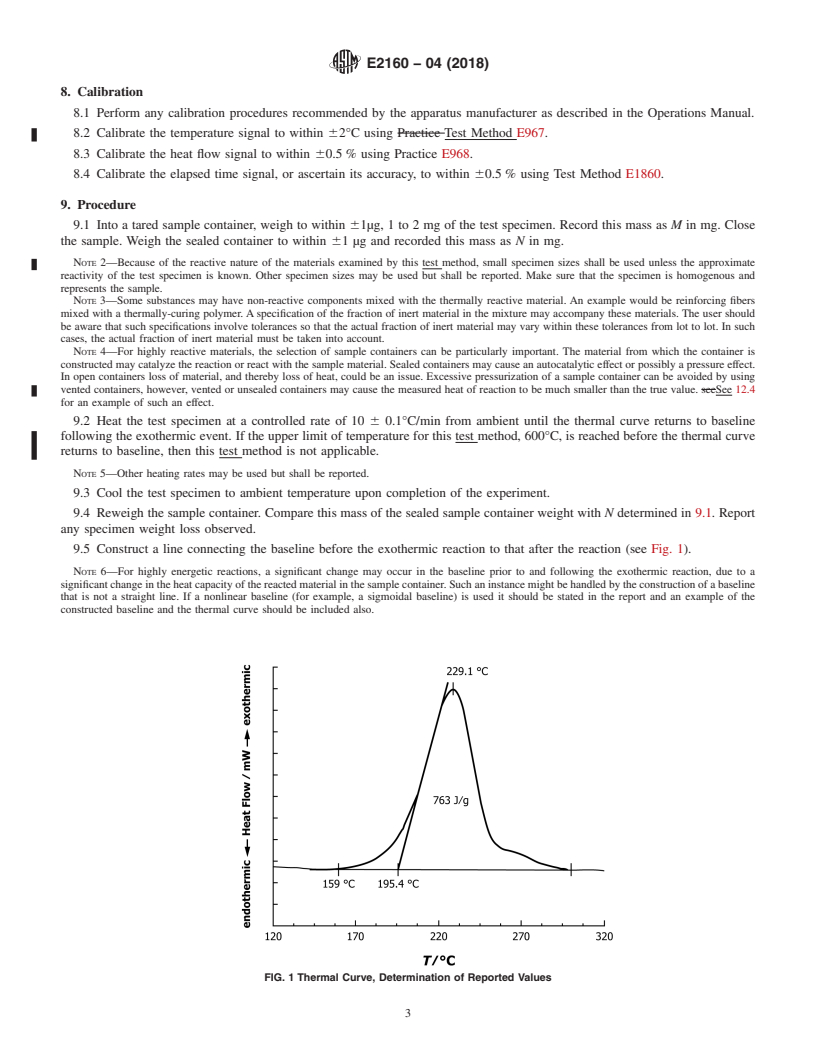
FIG. 1 Thermal Curve, Determination of Reported Values
E2160 − 04 (2018)
FIG. 2 Example of a Leaning Thermal Curve Resulting From Too
Much Material in the Sample Pan
10. Calculations of the test method and the second part examined interlabora-
tory differenced from confounding variables. the results of
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E2160 − 04 (Reapproved 2012) E2160 − 04 (Reapproved 2018)
Standard Test Method for
Heat of Reaction of Thermally Reactive Materials by
Differential Scanning Calorimetry
This standard is issued under the fixed designation E2160; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method determines the exothermic heat of reaction of thermally reactive chemicals or chemical mixtures, using
milligram specimen sizes, by differential scanning calorimetry. Such reactive materials may include thermally unstable or
thermoset materials.
1.2 This test method also determines the extrapolated onset temperature and peak heat flow temperature for the exothermic
reaction.
1.3 This test method may be performed on solids, liquids or slurries.
1.4 The applicable temperature range of this test method is 25 to 600°C.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 There is no ISO method equivalent to this standard.
1.7 This standard is related to Test Method E537 and to NAS 1613, but provides additional information.
1.8 This standard may involve hazardous materials, operations, and equipment. This standard does not purport to address all
of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate
safety safety, health, and healthenvironmental practices and determine the applicability of regulatory limitations prior to use.
1.9 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 ASTM Standards:
E473 Terminology Relating to Thermal Analysis and Rheology
E537 Test Method for The Thermal Stability of Chemicals by Differential Scanning Calorimetry
E967 Test Method for Temperature Calibration of Differential Scanning Calorimeters and Differential Thermal Analyzers
E968 Practice for Heat Flow Calibration of Differential Scanning Calorimeters
E1142 Terminology Relating to Thermophysical Properties
E1231 Practice for Calculation of Hazard Potential Figures of Merit for Thermally Unstable Materials
E1860 Test Method for Elapsed Time Calibration of Thermal Analyzers
2.2 Other Standard:
NAS 1613 Seal Element, Packing, Preformed, Ethylene Propylene Rubber, RubberNational Aerospace Standard, Aerospace
Industries Association of America, 1725 DeSales St., NM, Washington, DC 20036
3. Terminology
3.1 Specific technical terms used in this standard are defined in Terminologies E473 and E1142.
This test method is under the jurisdiction of ASTM Committee E37 on Thermal Measurements and is the direct responsibility of Subcommittee E37.01 on Calorimetry
and Mass Loss.
Current edition approved Sept. 1, 2012April 1, 2018. Published September 2012May 2018. Originally approved in 2001. Last previous edition approved in 20042012 as
E2160 – 04.E2160 – 04 (2012). DOI: 10.1520/E2160-04R12.10.1520/E2160-04R18.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Available from National Aerospace Standard (NAS), Aerospace Industries Association (AIA), 1000 Wilson Blvd., Suite 1700, Arlington, VA 22209, http://www.aia-
aerospace.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2160 − 04 (2018)
4. Summary of Test Method
4.1 A small (milligram) quantity of the reactive material is heated at 10°C/min through a temperature region where a chemical
reaction takes place. The exothermic heat flow produced by the reaction is recorded as a function of temperature and time by a
differential scanning calorimeter. Integration of the exothermic heat flow over time yields the heat of reaction. If the heat flow is
endothermic, then this test method is not to be used.
4.2 The test method can be used to determine the fraction of a reaction that has occurred in a partially reacted sample. The heat
of reaction is determined for a specimen that is known to be 100 % unreacted and is compared to the heat of reaction determined
for the partially reacted sample. Appropriate calculation yields the fraction of the latter sample that was unreacted.
4.3 Subtracting the reaction fraction remaining from unity (1) yields the fraction reacted. The fraction reacted may be expressed
as percent. If the sample tested is a thermoset resin, the percent reacted is often called the percent of cure.
4.4 The extrapolated onset temperature and peak heat flow temperature are determined for the exothermic heat flow thermal
curve from 4.1.
5. Significance and Use
5.1 This test method is useful in determining the extrapolated onset temperature, the peak heat flow temperature and the heat
of reaction of a material. Any onset temperature determined by this test method is not valid for use as the sole information used
for determination of storage or processing conditions.
5.2 This test method is useful in determining the fraction of a reaction that has been completed in a sample prior to testing. This
fraction of reaction that has been completed can be a measure of the degree of cure of a thermally reactive polymer or can be a
measure of decomposition of a thermally reactive material upon aging.
5.3 The heat of reaction values may be used in Practice E1231 to determine hazard potential figures-of-merit Explosion
Potential and Shock Sensitivity.
5.4 This test method may be used in research, process control, quality assurance, and specification acceptance.
6. Apparatus
6.1 Differential Scanning Calorimeter (DSC), capable of measuring and recording heat flow as a function of temperature and
time. Such a DSC is composed of:
6.1.1 Test Chamber, composed of:
6.1.1.1 Furnace(s), to provide uniform controlled heating of a specimen and reference to a constant temperature or at a constant
rate within the temperature range of 25 to 600°C.
6.1.1.2 Temperature Sensor, to provide an indication of the specimen or furnace temperature to within 60.5°C.
6.1.1.3 Differential Sensor, to detect a heat flow difference between the specimen and reference equivalent to 0.2 mW.
6.1.1.4 Means of Sustaining a Test Chamber Environment, of inert (for example, nitrogen, helium or argon) or reactive (for
example, air) gas at a purge rate of 50 6 5 mL/min.
NOTE 1—Typically, at least 99 % pure nitrogen, helium or argon is employed when oxidation in air is a concern. Unless effects of moisture are to be
studied, use of dry purge gas is recommended.
6.1.1.5 Temperature Controller, capable of executing a specific temperature program by operating the furnace(s) between
selected temperature limits (ambient temperature to 600°C) at a heating rate between 2 and 20°C/min constant to within
60.1°C/min.
6.1.1.6 Recording Device, capable of recording and displaying any portion (including signal noise) of the differential heat flow
on the ordinate as a function of temperature or time on the abscissa.
6.2 Containers, (pans, crucibles, vials, etc. and lids) that are inert to the specimen and reference materials and that are of suitable
structural shape and integrity to contain the specimen and reference in accordance with the specific requirements of this test
method.
6.3 Balance, with a capacity of 100 mg or greater to weigh specimens and containers, or both, to a sensitivity of 61 μg.
7. Safety Precautions
7.1 The use of this test method for materials of unknown potential hazards requires that precautions be taken during the sample
preparation and testing.
7.2 Where particle size reduction by grinding is necessary, the user of this test method shall presume that the material is
hazardous.
7.3 Toxic or corrosive effluents, or both, may be released when heating the test specimen and could be harmful to personnel
or the apparatus. Use of an exhaust system to remove such effluents is recommended.
E2160 − 04 (2018)
8. Calibration
8.1 Perform any calibration procedures recommended by the apparatus manufacturer as described in the Operations Manual.
8.2 Calibrate the temperature signal to within 62°C using Practice Test Method E967.
8.3 Calibrate the heat flow signal to within 60.5 % using Practice E968.
8.4 Calibrate the elapsed time signal, or ascertain its accuracy, to within 60.5 % using Test Method E1860.
9. Procedure
9.1 Into a tared sample container, weigh to within 61μg, 1 to 2 mg of the test specimen. Record this mass as M in mg. Close
the sample. Weigh the sealed container to within 61 μg and recorded this mass as N in mg.
NOTE 2—Because of the reactive nature of the materials examined by this test method, small specimen sizes shall be used unless the approximate
reactivity of the test specimen is known. Other specimen sizes may be used but shall be reported. Make sure that the specimen is homogenous and
represents the sample.
NOTE 3—Some substances may have non-reactive components mixed with the thermally reactive material. An example would be reinforcing fibers
mixed with a thermally-curing polymer. A specification of the fraction of inert material in the mixture may accompany these materials. The user should
be aware that such specifications involve tolerances so that the actual fraction of inert material may vary within these tolerances from lot to lot. In such
cases, the actual fraction of inert material must be taken into account.
NOTE 4—For highly reactive materials, the selection of sample containers can be particularly important. The material from which the container is
constructed may catalyze the reaction or react with the sample material. Sealed containers may cause an autocatalytic effect or possibly a pressure effect.
In open containers loss of material, and thereby loss of heat, could be an issue. Excessive pressurization of a sample container can be avoided by using
vented containers, however, vented or unsealed containers may cause the measured heat of reaction to be much smaller than the true value. seeSee 12.4
for an example of such an effect.
9.2 Heat the test specimen at a controlled rate of 10 6 0.1°C/min from ambient until the thermal curve returns to baseline
following the exothermic event. If the upper limit of temperature for this test method, 600°C, is reached before the thermal curve
returns to baseline, then this test method is not applicable.
NOTE 5—Other heating rates may be used but shall be reported.
9.3 Cool the test specimen to ambient temperature upon completion of the experiment.
9.4 Reweigh the sample container. Compare this mass of the sealed sample container weight with N determined in 9.1. Report
any specimen weight loss observed.
9.5 Construct a line connecting the baseline before the exothermic reaction to that after the reaction (see Fig. 1).
NOTE 6—For highly energetic reactions, a significant change may occur in the baseline prior to and following the exothermic reaction, due to a
significant change in the heat capacity of the reacted material in the sample container. Such an instance might be handled by the construction of a baseline
that is not a straight line. If a nonlinear baseline (for example, a sigmoidal baseline) is used it should be stated in the report and an example of the
constructed baseline and the thermal curve should be included also.
FIG. 1 Thermal Curve, Determination of Reported Values
E2160 − 04 (2018)
9.6 Integrate the area, as a function of time, bounded by the thermal curve and the baseline constructed in 9.5. Record this area
as the heat of the reaction (A) in mJ.
NOTE 7—The area bounded by the thermal curve and the constructed baseline gives the heat of the reaction. Instrument software is most often used
to integrate this area. Although such software packages display thermal curves as in Fig. 1, they calculate the bound area on a basis of time. If older
instruments without these software packages are used, or if manual checks are performed on newer instruments, then the manual integration must be
performed with the abscissa presented as a time (seconds) coordinate.
NOTE 8—The amount of material should be chosen such that the maximum heat flow is less than 8 mW. This requirement reduces the potential of
obtaining adiabatic heating of the sample. Adiabatic heating of the sample results in “leaning” peaks, an example of which is shown in Fig. 2 (adapted
from Figure 11 of Jones (1996)). . For highly energetic materials, it might be impossible to satisfy simultaneously the direction of 9.1 (using 1 to 2 mg
of the test specimen) and the condition of this note (maximum heat flow less than 8 mW). If heat flow is larger than 8 mW and the peak is not “leaning”,
it should not be necessary to reduce sample mass. Or, in other words, when both directions cannot be met simultaneously, sample mass need be reduced
only if the observe peak leans.
9.7 Construct a tangent to the leading edge of the exothermic peak at the point of maximum rate of change and extrapolate that
tangent to the baseline constructed in 9.5. Record the intersection of the tangent with the baseline as the onset temperature (To).
NOTE 9—In some cases, reactions may have induction periods or other effects that are manifested as exothermic deviations from the established
baseline well before the onset temperature obtained by 9.7. Because of the importance of these effects for highly reactive materials, an additional onset
temperature, the temperature of first deviation (Tf), is to be reported also. The temperature of first deviation is the temperature for which the thermal curve
first deviates from the established baseline. The temperature of first deviation is to be noted in the report.
NOTE 10—Peak temperatures from two different determinations are comparable only if the same conditions were used for both measurements, for
example, sample mass and vent di
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.