ASTM D3612-02(2009)
(Test Method)Standard Test Method for Analysis of Gases Dissolved in Electrical Insulating Oil by Gas Chromatography
Standard Test Method for Analysis of Gases Dissolved in Electrical Insulating Oil by Gas Chromatography
SIGNIFICANCE AND USE
Oil and oil-immersed electrical insulation materials may decompose under the influence of thermal and electrical stresses, and in doing so, generate gaseous decomposition products of varying composition which dissolve in the oil. The nature and amount of the individual component gases that may be recovered and analyzed may be indicative of the type and degree of the abnormality responsible for the gas generation. The rate of gas generation and changes in concentration of specific gases over time are also used to evaluate the condition of the electric apparatus.
Note 1—Guidelines for the interpretation of gas-in-oil data are given in IEEE C57.104.
SCOPE
1.1 This test method covers three procedures for extraction and measurement of gases dissolved in electrical insulating oil having a viscosity of 20 cSt (100 SUS) or less at 40°C (104°F), and the identification and determination of the individual component gases extracted. Other methods have been used to perform this analysis.
1.2 The individual component gases that may be identified and determined include:
Hydrogen—H2 Oxygen—O2 Nitrogen—N2 Carbon monoxide—CO Carbon dioxide—CO2 Methane—CH4 Ethane—C2H6 Ethylene—C2H4 Acetylene—C2H2 Propane—C3H8 Propylene—C3H6
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific warning statements see 6.1.8, 30.2.2 and 30.3.1.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D3612 − 02 (Reapproved 2009)
Standard Test Method for
Analysis of Gases Dissolved in Electrical Insulating Oil by
Gas Chromatography
This standard is issued under the fixed designation D3612; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D2779Test Method for Estimation of Solubility of Gases in
Petroleum Liquids
1.1 This test method covers three procedures for extraction
D2780TestMethodforSolubilityofFixedGasesinLiquids
and measurement of gases dissolved in electrical insulating oil
(Withdrawn 2010)
havingaviscosityof20cSt(100SUS)orlessat40°C(104°F),
D3613Practice for Sampling Insulating Liquids for Gas
and the identification and determination of the individual
AnalysisandDeterminationofWaterContent(Withdrawn
component gases extracted. Other methods have been used to
2007)
perform this analysis.
D4051PracticeforPreparationofLow-PressureGasBlends
1.2 The individual component gases that may be identified
E260Practice for Packed Column Gas Chromatography
and determined include:
2.2 IEEE Standard:
Hydrogen—H
2 C57.104 GuidefortheInterpretationofGasesGeneratedin
Oxygen—O 4
Oil-Immersed Transformers
Nitrogen—N
2.3 IEC Standard:
Carbon monoxide—CO
Carbon dioxide—CO
2 PublicationNo.567GuidefortheSamplingofGasesandof
Methane—CH
Oil from Oil-Filled Electrical Equipment and for the
Ethane—C H
2 6
Analysis of Free and Dissolved Gases
Ethylene—C H
2 4
Acetylene—C H
2 2
Propane—C H
3. Terminology
3 8
Propylene—C H
3 6
3.1 Definitions of Terms Specific to This Standard:
1.3 This standard does not purport to address all of the
3.1.1 gas content of oil by volume—in Method A, the total
safety concerns, if any, associated with its use. It is the
volume of gases, corrected to 760 torr (101.325 kPa) and 0°C,
responsibility of the user of this standard to establish appro-
contained in a given volume of oil, expressed as a percentage.
priate safety and health practices and determine the applica-
In Methods B and C, the sum of the individual gas concentra-
bility of regulatory limitations prior to use. For specific
tionscorrectedto760torr(101.325kPa)and0°C,expressedin
warning statements see 6.1.8, 30.2.2 and 30.3.1.
percent or parts per million.
3.1.2 headspace—a volume of gas phase in contact with a
2. Referenced Documents
volumeofoilinaclosedvessel.Thevesselisaheadspacevial
2.1 ASTM Standards:
of 20-mL nominal capacity.
D2140Practice for Calculating Carbon-Type Composition
3.1.2.1 Discussion—Other vessel volumes may also be
of Insulating Oils of Petroleum Origin
used, but the analytical performance may be somewhat differ-
D2300Test Method for Gassing of Electrical Insulating
ent than that specified in Method C.
Liquids Under Electrical Stress and Ionization (Modified
3.1.3 parts per million (ppm) by volume of (specific gas) in
Pirelli Method)
oil—thevolumeofthatgascorrectedto760torr(101.325kPa)
and 0°C, contained in 10 volume of oil.
3.1.4 sparging, v—agitatingtheliquidsampleusingagasto
This test method is under the jurisdiction of ASTM Committee D27 on
Electrical Insulating Liquids and Gasesand is the direct responsibility of Subcom-
strip other gases free.
mittee D27.03 on Analytical Tests.
Current edition approved May 15, 2009. Published June 2009. Originally
approved in 1977. Last previous edition approved in 2002 as D3612–02 (2009). The last approved version of this historical standard is referenced on
DOI: 10.1520/D3612-02R09. www.astm.org.
2 4
For referenced ASTM standards, visit the ASTM website, www.astm.org, or Available from IEEE, 345 E. 47th St., New York, NY 10017.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Available from International Electrotechnical Commission (IEC), 3 rue de
Standards volume information, refer to the standard’s Document Summary page on Varembé, Case postale 131, CH-1211, Geneva 20, Switzerland, http://www.iec.ch.
the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3612 − 02 (2009)
3.1.5 volume concentration of (specific gas) in the gas 6. Apparatus
sample—the volume of the specific gas contained in a given 6
6.1 Apparatus of the type shown in Fig. 1 or Fig. 2 is
volumeofthegassampleatthesametemperatureandpressure
suitable for use with up to 50-mL samples of oil and consists
(as the measured total volume), expressed either as a percent-
of the following components:
age or in parts per million.
NOTE2—Thissamplesizehasbeenfoundtobesufficientformostoils.
However, oil that has had only limited exposure to air may contain much
4. Summary of Test Method
smalleramountsofnitrogenandoxygen.Fortheseoilsitmaybedesirable
to increase the size of the sample and the extraction apparatus.
4.1 MethodA—Dissolvedgasesareextractedfromasample
NOTE 3—Alternative apparatus designs including the use of a Toepler
of oil by introduction of the oil sample into a pre-evacuated
pump have also been found successful.
known volume. The evolved gases are compressed to atmo-
6.1.1 Polytetrafluoroethylene (PTFE) Tubing, narrow-bore,
spheric pressure and the total volume measured.
terminatedwithaLuer-Lockfittedglasssyringe,andleadingto
a solid plug, three-way, high-vacuum stopcock.
4.2 Method B—Dissolvedgasesareextractedfromasample
6.1.2 Degassing Flask, with a glass inlet tube, of sufficient
of oil by sparging the oil with the carrier gas on a stripper
volume to contain up to 50 mL of oil below the inlet tube,
column containing a high surface area bead.
capable of being evacuated through a vacuum pump, contain-
4.3 Method C—MethodCconsistsofbringinganoilsample
ing a PTFE-coated magnetic spin bar, and mounted on a
in contact with a gas phase (headspace) in a closed vessel
magnetic stirrer.
purgedwithargon.Thedissolvedgasescontainedintheoilare
6.1.3 Means of Measuring Absolute Pressure within the
then equilibrated in the two phases in contact under controlled
apparatus.
conditions (in accordance with Henry’s law). At equilibrium,
6.1.4 Vacuum Pumping System, capable of evacuating the
−3
the headspace is overpressurized with argon and then the
glasswaretoanabsolutepressureof1×10 torr(130mPa)or
content of a loop is filled by the depressurization of the
lower.
headspaceagainsttheambientatmosphericpressure.Thegases
6.1.5 Vacuum Glassware, sufficiently large compared to the
contained in the loop are then introduced into a gas chromato-
volume of the oil sample, so that virtually complete degassing
graph.
isobtainedandthatthevolumetriccollectionratioisaslargeas
possible. A 500-mL gas collecting flask has been found
4.4 Theremaybesomedifferencesinthelimitsofdetection
suitable.
and precision and bias between Methods A, B, and C for
6.1.6 High-Vacuum Valves or Stopcocks, employing the
various gases.
minimum necessary amounts of high-vacuum stopcock grease
4.5 Aportionoftheextractedgases(MethodA)orallofthe
are used throughout the apparatus.
extractedgases(MethodB)oraportionoftheheadspacegases
6.1.7 Gas Collection Tube, calibrated in 0.01-mLdivisions,
(Method C) is introduced into a gas chromatograph. Calibra-
capable of containing up to 5 mL of gas, terminated with a
tioncurvesareusedinMethodCtoestablishtheconcentration
silicone rubber retaining septum. A suitable arrangement is
of each species. The composition of the sample is calculated shown in Fig. 3.
from its chromatogram by comparing the area of the peak of
6.1.8 Reservoir of Mercury, sufficient to fill the collection
each component with the area of the peak of the same flask and collection tube. (Warning—Mercury vapor is ex-
component on a reference chromatogram made on a standard
tremely toxic. Appropriate precautions should be taken.)
mixture of known composition.
7. Sampling
5. Significance and Use 7.1 Obtain samples in accordance with the procedure de-
scribed in Test Methods D3613 for sampling with syringetype
5.1 Oilandoil-immersedelectricalinsulationmaterialsmay
devices or rigid metal cylinders. The use of rigid metal
decompose under the influence of thermal and electrical
cylinders is not recommended for use with Method B.
stresses, and in doing so, generate gaseous decomposition
7.2 The procurement of representative samples without loss
products of varying composition which dissolve in the oil.The
ofdissolvedgasesorexposuretoairisveryimportant.Itisalso
natureandamountoftheindividualcomponentgasesthatmay
important that the quantity and composition of dissolved gases
be recovered and analyzed may be indicative of the type and
remain unchanged during transport to the laboratory. Avoid
degree of the abnormality responsible for the gas generation.
prolonged exposure to light by immediately placing drawn
The rate of gas generation and changes in concentration of
specific gases over time are also used to evaluate the condition
of the electric apparatus.
Ace Glass and Lurex Glass manufacture glass extractors. For Ace Glass, the
glass apparatus conforming to Fig. 1 is Part E-13099-99-99 and Fig. 2 is Part
NOTE1—Guidelinesfortheinterpretationofgas-in-oildataaregivenin
E-1400-99. Available from P.O. Box 688, 1430 Northwest Blvd., Vineland, NJ
IEEE C57.104. 08360 or Lurex Glass, 1298 Northwest Blvd., Vineland, NJ 08360.
D3612 − 02 (2009)
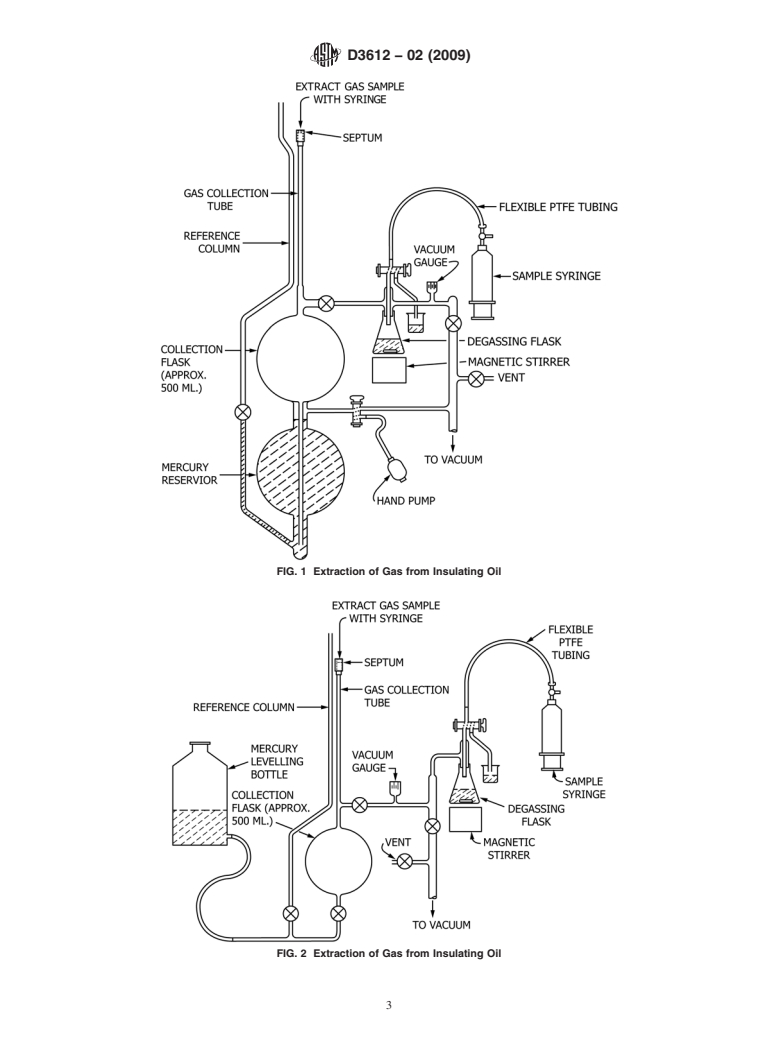
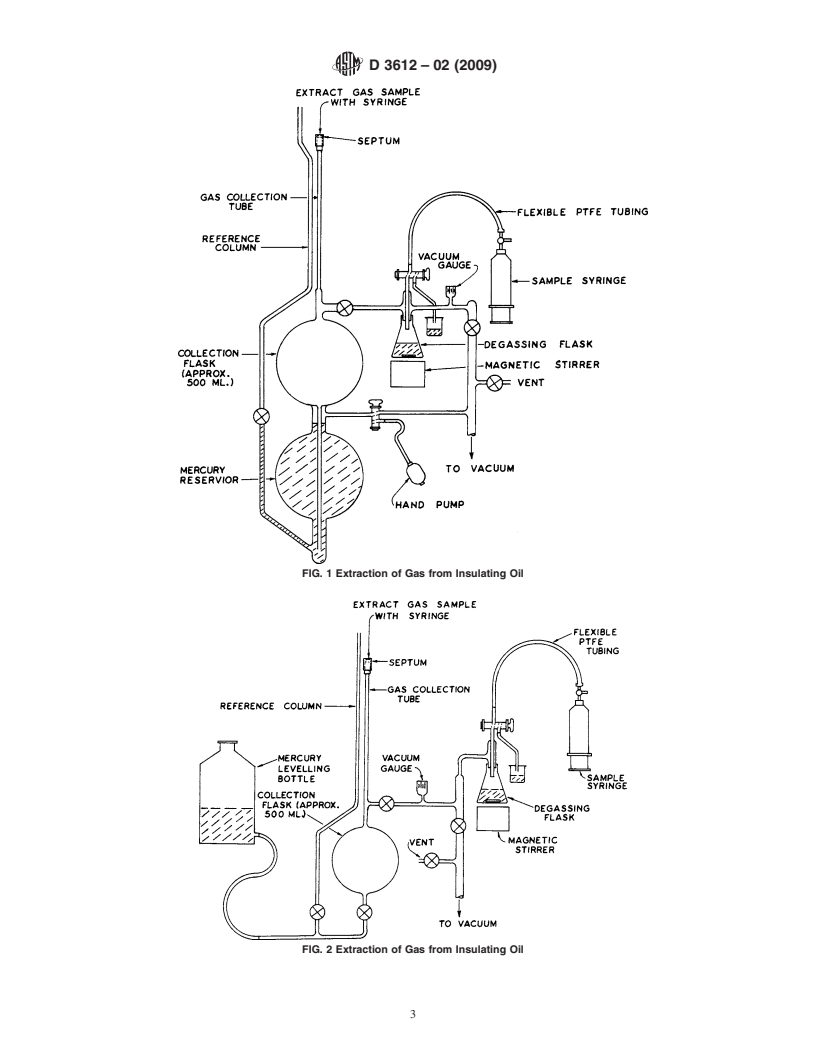
FIG. 1 Extraction of Gas from Insulating Oil
FIG. 2 Extraction of Gas from Insulating Oil
D3612 − 02 (2009)
V = totalinternalvolumeofextractionapparatusbeforeoil
T
sample is introduced, and
K = Ostwald solubility coefficient of component i.
i
9.4 Determine the Ostwald solubility coefficients of fixed
gases in accordance with Test Method D2780.
9.5 Ostwald solubility coefficients that have been deter-
mined for a number of gases in one specific electrical insulat-
ing oil at 25°C are shown as follows.Values for gases in other
oils may be estimated by reference to Test Method D2779.
Ostwald Solubility (Note 5)
Component Gas
Coefficient, K , 25°C, 760 mm Hg
i
Hydrogen 0.0558
Nitrogen 0.0968
Carbon monoxide 0.133
Oxygen 0.179
Methane 0.438
Carbon dioxide 1.17
Acetylene 1.22
Ethylene 1.76
FIG. 3 Retaining Rubber Septum for Gas Collection Tube
Ethane 2.59
Propane 11.0
NOTE 5—The Ostwald coefficient values shown in this table are correct
onlyforthespecificmineraloilhavingadensityat15.5°Cof0.855g/cm
samples into light-proof containers and retaining them there
usedintheoriginaldetermination.Ostwaldcoefficientsformineraloilsof
until the start of testing.
different density may be calculated as follows:
7.2.1 To maintain the integrity of the sample, keep the time
0.980 2density
between sampling and testing as short as possible. Evaluate
K ~corrected! 5 K (3)
i i
0.130
containers for maximum storage time. Samples have been
where, density =density of the oil of interest, g/cm at 15.5°C (60°F).
stored in syringes and metal cylinders for four weeks with no
This equation is derived from the equation in Test Method D2779. Note
appreciable change in gas content.
especially that all of the Ostwald coefficients are changed by the same
factor, meaning that though the absolute solubilities of each of the gases
NOTE 4—Additional sampling procedures using flexible metal cans are
will change if a different oil is used, the ratio of the solubility of one gas
currently being studied for use with Method A.
to another gas will remain constant.
METHOD A—VACUUM EXTRACTION
9.6 A procedure to check the extraction efficiency requires
the use of prepared gas-in-oil standards of known concentra-
8. Method A—Vacuum Extraction
tion.ThemethodsofpreparationareoutlinedinAnnexA1and
Annex A2.
8.1 Method A employs vacuum extraction to separate the
gases from the oil. The evolved gases are compressed to
10. Procedure
atmospheric pressure and the total volume measured. The
10.1 Lower the mercury level from the collection flask.
gases are then analyzed by gas chromatography.
10.2 Evacuate the system of collection flask and degassing
9. Preparation of Apparatus −3
flasktoanabsolutepressureof1×10 torr(130mPa)orless.
(In Fig. 1, the space above the mercury in the reservoir must
9.1 Check the apparatus carefully for vacuum tightness of
all joints and stopcocks. also be evacuated.)
10.3 Connect the oil sample syringe by the PTFE tubing to
9.2 Measure the total volume of the extraction apparatus,
V , and the volume of the collection space, V , and calculate the three-way stopcock leading to the degassing flask.
T c
the ratio as the volumetric collection ratio:
10.4 Flush a small quantity of oil from the syringe through
V thetubingandstopcocktowaste,makingsurethatalltheairin
c
(1)
V 2 V the connecting tubing is displaced by oil.
T o
10.4.1 Any gas bubbles present in the syringe should be
where V =the volume of oil to be added.
o
retained during this flushing operation. This may be accom-
9.3 Calculate the degassing efficiencies for each individual
plished by inverting the syringe so that the bubble remains at
component gas as follows: the plunger end of the syringe during the flushing operation.
10.5 Close the stopcocks to the vacuum pumps and then
E 5 (2)
i
K V
slowly open the three-way stopcock to allow oil and any gas
i o
V 2 V
bubbles that may be present from the sample syringe to enter
T o
the degassing flask.
where:
E = degassing efficiency of component i,
i
Daoust, R., Dind, J. E., Morgan, J., and Regis, J, “Analysis of Gas Dissolved
V = volume of oil sample,
o
in Transformer Oils,” Doble Conference, 1971, Sections 6–110.
D3612 − 02 (2009)
10.6 Allow the desired amount of oil to enter the degassing 11.4 The apparatus shall provide sufficient repeatability so
flask and operate the magnetic stirrer vigorously for approxi- thatsuccessiverunsofareferencestandardagreewithin 61%
mately 10 min. This is the volume, V used in the calculation with respect to area under the peaks for hydrocarbon and
o
in 15.4. carbon oxide components.
10.6.1 If a gas bubble is present in the syringe, either
11.5 Awiderangeofchromatographicconditionshavebeen
analyze the total content of the syringe including the bubble;
successfullyemployed.Bothargonandheliumhavebeenused
or, if the gas bubble is large, and it is suspected that the
as carrier gases (see Note 7). In some cases, a separate GC or
concentration of dissolved gases is high, measure and analyze
other device is used for the detection and quantification of
the gas bubble separately, extract an aliquot of the oil sample,
hydrogen when helium is used as a carrier gas.
and correct as applicable.
...
This document is not anASTM standard and is intended only to provide the user of anASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation:D 3612–01 Designation:D 3612–02 (Reapproved 2009)
Standard Test Method for
Analysis of Gases Dissolved in Electrical Insulating Oil by
Gas Chromatography
This standard is issued under the fixed designation D 3612; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers three procedures for extraction and measurement of gases dissolved in electrical insulating oil
having a viscosity of 20 cSt (100 SUS) or less at 40°C (104°F), and the identification and determination of the individual
component gases extracted. Other methods have been used to perform this analysis.
1.2 The individual component gases that may be identified and determined include:
Hydrogen—H
Oxygen—O
Nitrogen—N
Carbon monoxide—CO
Carbon dioxide—CO
Methane—CH
Ethane—C H
2 6
Ethylene—C H
2 4
Acetylene—C H
2 2
Propane—C H
3 8
Propylene—C H
3 6
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use. For specific precautionarywarning statements see 6.1.8, 30.2.2 and 30.3.1.
2. Referenced Documents
2.1 ASTM Standards:
D2140 Test Method Practice for Calculating Carbon-Type Composition of Insulating Oils of Petroleum Origin
D2300 Test Method for Gassing of Electrical Insulating OilsLiquids Under Electrical Stress and Ionization (Modified Pirelli
Method Method)
D2779 Test Method for Estimation of Solubility of Gases in Petroleum Liquids
D2780 Test Method for Solubility of Fixed Gases in Liquids
D3613 Test Methods of Sampling Electrical Insulating Oils for Gas Analysis and Determination of Water Content
Practice for Sampling Insulating Liquids for Gas Analysis and Determination of Water Content
D4051 Practice for Preparation of Low-Pressure Gas Blends
E260 Practice for Packed Column Gas Chromatography
2.2 IEEE Standard:
C57.104 Guide for the Interpretation of Gases Generated in Oil-Immersed Transformers
2.3 IEC Standard:
Publication No. 567 Guide for the Sampling of Gases and of Oil from Oil-Filled Electrical Equipment and for theAnalysis of
Free and Dissolved Gases
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
This test method is under the jurisdiction ofASTM Committee D27 on Electrical Insulating Liquids and Gases and is the direct responsibility of Subcommittee D27.03
on Analytical Tests.
Current edition approved Feb. 10, 2001. Published April 2001. Originally published as D3612–77. Last previous edition D3612–96.
Current edition approved May 15, 2009. Published June 2009. Originally approved in 1977. Last previous edition approved in 2002 as D3612–02 (2009).
Annual Book of ASTM Standards, Vol 05.02.
Available from IEEE, 345 E. 47th St., New York, NY 10017.
Annual Book of ASTM Standards, Vol 14.02.
Available from International Electrotechnical Commission (IEC), 3 rue de Varembé, Case postale 131, CH-1211, Geneva 20, Switzerland, http://www.iec.ch.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 3612–02 (2009)
3.1.1 gas content of oil by volume— in Method A, the total volume of gases, corrected to 760 torr (101.325 kPa) and 0°C,
containedinagivenvolumeofoil,expressedasapercentage.InMethodsBandC,C,thesumoftheindividualgasconcentrations
corrected to 760 torr (101.325 kPa) and 0°C, expressed in percent or parts per million.
3.1.2 headspace—a volume of gas phase in contact with a volume of oil in a closed vessel. The vessel is a headspace vial of
20-mL nominal capacity.
3.1.2.1 Discussion—Other vessel volumes may also be used, but the analytical performance may be somewhat different than
that specified in Method C.
3.1.3 parts per million (ppm) by volume of (specific gas) in oil—the volume of that gas corrected to 760 torr (101.325 kPa) and
0°C, contained in 10 volume of oil.
3.1.4 sparging, v—agitating the liquid sample using a gas to strip other gases free.
3.1.5 volume concentration of (specific gas) in the gas sample—the volume of the specific gas contained in a given volume of
the gas sample at the same temperature and pressure (as the measured total volume), expressed either as a percentage or in parts
per million.
4. Summary of Test Method
4.1 MethodA—Dissolvedgasesareextractedfromasampleofoilbyintroductionoftheoilsampleintoapre-evacuatedknown
volume. The evolved gases are compressed to atmospheric pressure and the total volume measured.
4.2 Method B—Dissolved gases are extracted from a sample of oil by sparging the oil with the carrier gas on a stripper column
containing a high surface area bead.
4.3 Method C—Method C consists of bringing an oil sample in contact with a gas phase (headspace) in a closed vessel purged
with argon.The dissolved gases contained in the oil are then equilibrated in the two phases in contact under controlled conditions
(in accordance with Henry’s law).At equilibrium, the headspace is overpressurized with argon and then the content of a loop is
filled by the depressurization of the headspace against the ambient atmospheric pressure.The gases contained in the loop are then
introduced into a gas chromatograph.
4.4 There may be some differences in the limits of detection and precision and bias between MethodsA, B, and C for various
gases.
4.5 A portion of the extracted gases (Method A) or all of the extracted gases (Method B) or a portion of the headspace gases
(MethodC)isintroducedintoagaschromatograph.CalibrationcurvesareusedinMethodCtoestablishtheconcentrationofeach
species.Thecompositionofthesampleiscalculatedfromitschromatogrambycomparingtheareaofthepeakofeachcomponent
withtheareaofthepeakofthesamecomponentonareferencechromatogrammadeonastandardmixtureofknowncomposition.
5. Significance and Use
5.1 Oil and oil-immersed electrical insulation materials may decompose under the influence of thermal and electrical stresses,
andindoingso,generategaseousdecompositionproductsofvaryingcompositionwhichdissolveintheoil.Thenatureandamount
oftheindividualcomponentgasesthatmayberecoveredandanalyzedmaybeindicativeofthetypeanddegreeoftheabnormality
responsibleforthegasgeneration.Therateofgasgenerationandchangesinconcentrationofspecificgasesovertimearealsoused
to evaluate the condition of the electric apparatus.
NOTE 1—Guidelines for the interpretation of gas-in-oil data are given in IEEE C57.104.
6. Apparatus
6.1Apparatus
6.1 Apparatus of the type shown in Fig. 1 or Fig. 2 is suitable for use with up to 50-mL samples of oil and consists of the
following components:
NOTE 2—This sample size has been found to be sufficient for most oils. However, oil that has had only limited exposure to air may contain much
smaller amounts of nitrogen and oxygen. For these oils it may be desirable to increase the size of the sample and the extraction apparatus.
NOTE 3—Alternative apparatus designs including the use of a Toepler pump have also been found successful.
6.1.1 Polytetrafluoroethylene (PTFE) Tubing, narrow-bore, terminated with a Luer-Lock fitted glass syringe, and leading to a
a solid plug, three-way, high-vacuum stopcock.
6.1.2 Degassing Flask, with a glass inlet tube, of sufficient volume to contain up to 50 mLof oil below the inlet tube, capable
of being evacuated through a vacuum pump, containing a PTFE-coated magnetic spin bar, and mounted on a magnetic stirrer.
6.1.3 Means of Measuring Absolute Pressure within the apparatus.
−3
6.1.4 Vacuum Pumping System, capable of evacuating the glassware to an absolute pressure of 1 310 torr (130 mPa) or
lower.
Available from IEEE, 345 E. 47th St., New York, NY 10017.
Ace Glass and Lurex Glass manufacture glass extractors. ForAce Glass, the glass apparatus conforming to Fig. 1 is Part E-13099-99-99 and Fig. 2 is Part E-1400-99.
Available from P.O. Box 688, 1430 Northwest Blvd., Vineland, NJ 08360 or Lurex Glass, 1298 Northwest Blvd., Vineland, NJ 08360.
D 3612–02 (2009)
FIG. 1 Extraction of Gas from Insulating Oil
FIG. 2 Extraction of Gas from Insulating Oil
D 3612–02 (2009)
6.1.5 Vacuum Glassware, sufficiently large compared to the volume of the oil sample, so that virtually complete degassing is
obtained and that the volumetric collection ratio is as large as possible. A 500-mL gas collecting flask has been found suitable.
6.1.6 High-Vacuum Valves or Stopcocks,employingtheminimumnecessaryamountsofhigh-vacuumstopcockgreaseareused
throughout the apparatus.
6.1.7 Gas Collection Tube, calibrated in 0.01-mLdivisions, capable of containing up to 5 mLof gas, terminated with a silicone
rubber retaining septum. A suitable arrangement is shown in Fig. 3.
6.1.8 Reservoir of Mercury, sufficient to fill the collection flask and collection tube. Note4—(Caution:Mercury Warning
—Mercury vapor is extremely toxic. Appropriate precautions should be taken.)
7. Sampling
7.1 Obtain samples in accordance with the procedure described in Test Methods D3613for sampling with syringetype devices
or rigid metal cylinders. The use of rigid metal cylinders is not recommended for use with Method B.
7.2 The procurement of representative samples without loss of dissolved gases or exposure to air is very important. It is also
important that the quantity and composition of dissolved gases remain unchanged during transport to the laboratory. Avoid
prolonged exposure to light by immediately placing drawn samples into light-proof containers and retaining them there until the
start of testing.
7.2.1 To maintain the integrity of the sample, keep the time between sampling and testing as short as possible. Evaluate
containersformaximumstoragetime.Sampleshavebeenstoredinsyringesandmetalcylindersforfourweekswithnoappreciable
change in gas content.
NOTE 54—Additional sampling procedures using flexible metal cans are currently being studied for use with Method A.
METHOD A—VACUUM EXTRACTION
8. Method A—Vacuum Extraction
8.1 MethodAemploys vacuum extraction to separate the gases from the oil.The evolved gases are compressed to atmospheric
pressure and the total volume measured. The gases are then analyzed by gas chromatography.
9. Preparation of Apparatus
9.1 Check the apparatus carefully for vacuum tightness of all joints and stopcocks.
9.2 Measure the total volume of the extraction apparatus, V , and the volume of the collection space, V , and calculate the ratio
T c
as the volumetric collection ratio:
V
c
(1)
V 2 V
T o
where V =the volume of oil to be added.
o
9.3 Calculate the degassing efficiencies for each individual component gas as follows:
E 5 (2)
i
KV
i o
1 1
V 2 V
T o
FIG. 3 Retaining Rubber Septum for Gas Collection Tube
D 3612–02 (2009)
where:
E = degassing efficiency of component i,
i
V = volume of oil sample,
o
V = total internal volume of extraction apparatus before oil sample is introduced, and
T
K = Ostwald solubility coefficient of component i.
i
9.4 Determine the Ostwald solubility coefficients of fixed gases in accordance with Test Method D2780.
9.5 Ostwald solubility coefficients that have been determined for a number of gases in one specific electrical insulating oil at
25°C are shown as follows. Values for gases in other oils may be estimated by reference to Test Method D2779.
Ostwald Solubility (Note 6)
Component Gas
Coefficient, K , 25°C, 760 mm Hg
i
Ostwald Solubility (Note 5)
Component Gas
Coefficient, K , 25°C, 760 mm Hg
i
Hydrogen 0.0558
Nitrogen 0.0968
Carbon monoxide 0.133
Oxygen 0.179
Methane 0.438
Carbon dioxide 1.17
Acetylene 1.22
Ethylene 1.76
Ethane 2.59
Propane 11.0
NOTE6—The 5—The Ostwald coefficient values shown in this table are correct only for the specific mineral oil having a density at 15.5°C of 0.855
g/cm used in the original determination. Ostwald coefficients for mineral oils of different density may be calculated as follows:
0.980 2density
K ~corrected!5 K (3)
i i
0.130
where, density =density of the oil of interest, g/cm at 15.5°C (60°F). This equation is derived from the equation in Test Method D2779. Note
especiallythatalloftheOstwaldcoefficientsarechangedbythesamefactor,meaningthatthoughtheabsolutesolubilitiesofeachofthegaseswillchange
if a different oil is used, the ratio of the solubility of one gas to another gas will remain constant.
9.6 Aproceduretochecktheextractionefficiencyrequirestheuseofpreparedgas-in-oilstandardsofknownconcentration.The
methods of preparation are outlined in Annex A1 and Annex A2.
10. Procedure
10.1 Lower the mercury level from the collection flask.
−3
10.2 Evacuate the system of collection flask and degassing flask to an absolute pressure of 1 310 torr (130 mPa) or less. (In
Fig. 1, the space above the mercury in the reservoir must also be evacuated.)
10.3 Connect the oil sample syringe by the PTFE tubing to the three-way stopcock leading to the degassing flask.
10.4 Flush a small quantity of oil from the syringe through the tubing and stopcock to waste, making sure that all the air in the
connecting tubing is displaced by oil.
10.4.1 Any gas bubbles present in the syringe should be retained during this flushing operation. This may be accomplished by
inverting the syringe so that the bubble remains at the plunger end of the syringe during the flushing operation.
10.5 Close the stopcocks to the vacuum pumps and then slowly open the three-way stopcock to allow oil and any gas bubbles
that may be present from the sample syringe to enter the degassing flask.
10.6 Allow the desired amount of oil to enter the degassing flask and operate the magnetic stirrer vigorously for approximately
10 min. This is the volume, V used in the calculation in 15.4.
o
10.6.1 If a gas bubble is present in the syringe, either analyze the total content of the syringe including the bubble; or, if the
gas bubble is large, and it is suspected that the concentration of dissolved gases is high, measure and analyze the gas bubble
separately, extract an aliquot of the oil sample, and correct as applicable.
10.7 Close the stopcock isolating the collection flask, and allow mercury to flow into the collection flask.
10.8
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.