ASTM F2790-10(2019)e1
(Practice)Standard Practice for Static and Dynamic Characterization of Motion Preserving Lumbar Total Facet Prostheses
Standard Practice for Static and Dynamic Characterization of Motion Preserving Lumbar Total Facet Prostheses
SIGNIFICANCE AND USE
5.1 Facet Prosthesis Components—The facet replacement may comprise a variety of shapes and configurations. Its forms may include, but are not limited to, ball and socket articulating joints, joints having a free-floating or semi-constrained third body, metallic load-bearing surfaces, and spring and dampening mechanisms. Additionally, it may have a unilateral or bilateral design.
5.2 These test methods are designed to quantify the static and dynamic characteristics of different designs of FPs. The tests are conducted in vitro in order to allow for analysis of individual devices and comparison of the mechanical performance of multiple designs.
5.3 The loads applied to the FP may differ from the complex loading seen in vivo, and therefore, the results from these tests may not directly predict in vivo performance. The results, however, can be used to compare mechanical performance in different devices.
5.4 Fatigue testing in a simulated body fluid or saline may cause fretting, corrosion, or lubricate the interconnections and thereby affect the relative performance of tested devices. This test should be conducted in a 0.9 % saline environmental bath at 37°C at a maximum rate of 10 Hz for metallic devices and 2 Hz for non-metallic devices. Other test environments such as a simulated body fluid, a saline drip or mist, distilled water, other type of lubrication or dry could also be used with adequate justification. Likewise, alternative test frequencies may be used with adequate justification to ensure that they do not impact the device performance.
5.5 It is well known that the failure of materials is dependent upon stress, test frequency, surface treatments, and environmental factors. Therefore, when determining the effect of changing these parameters (for example, frequency, material, or environment), care should be taken to allow for appropriate interpretation of the results. In particular, it may be necessary to assess the influence of test frequency on dev...
SCOPE
1.1 This practice provides guidance for the static and dynamic testing of Lumbar Total Facet Prostheses (FPs). These implants are intended to allow motion and lend support to one or more functional spinal unit(s) through replacement of the natural facets.
1.2 These test methods are intended to provide a basis for the mechanical comparison among past, present, and future non-biologic FPs. These test methods allow comparison of devices with different methods of application to the lumbar spine. These test methods are intended to enable the user to mechanically compare devices and do not purport to provide performance standards for them.
1.3 These test methods describe static and dynamic tests by specifying load types and specific methods of applying these loads.
1.4 These test methods do not purport to address all clinically relevant failure modes for FPs, some of which will be device-specific. For example, these test methods do not address implant wear resistance under expected in vivo loads and motions. In addition, the biologic response to wear debris is not addressed in these test methods.
1.5 Requirements are established for measuring displacements and evaluating the stiffness of an FP.
1.6 Some devices may not be testable in all test configurations.
1.7 The values stated in SI units are to be regarded as the standard with the exception of angular measurements, which may be reported in terms of either degrees or radians.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.9 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Developme...
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
´1
Designation: F2790 − 10 (Reapproved 2019)
Standard Practice for
Static and Dynamic Characterization of Motion Preserving
Lumbar Total Facet Prostheses
This standard is issued under the fixed designation F2790; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
ε NOTE—Editorial corrections were made throughout in December 2019.
1. Scope priate safety, health, and environmental practices and deter-
mine the applicability of regulatory limitations prior to use.
1.1 This practice provides guidance for the static and
1.9 This international standard was developed in accor-
dynamictestingofLumbarTotalFacetProstheses(FPs).These
dance with internationally recognized principles on standard-
implants are intended to allow motion and lend support to one
ization established in the Decision on Principles for the
or more functional spinal unit(s) through replacement of the
Development of International Standards, Guides and Recom-
natural facets.
mendations issued by the World Trade Organization Technical
1.2 These test methods are intended to provide a basis for
Barriers to Trade (TBT) Committee.
the mechanical comparison among past, present, and future
non-biologic FPs. These test methods allow comparison of 2. Referenced Documents
devices with different methods of application to the lumbar 2
2.1 ASTM Standards:
spine. These test methods are intended to enable the user to
D638 Test Method for Tensile Properties of Plastics
mechanically compare devices and do not purport to provide
E4 Practices for Force Calibration and Verification of Test-
performance standards for them.
ing Machines
1.3 These test methods describe static and dynamic tests by E6 Terminology Relating to Methods of Mechanical Testing
specifying load types and specific methods of applying these E468 Practice for Presentation of Constant Amplitude Fa-
loads. tigue Test Results for Metallic Materials
E739 PracticeforStatisticalAnalysisofLinearorLinearized
1.4 These test methods do not purport to address all clini-
Stress-Life (S-N) and Strain-Life (ε-N) Fatigue Data
cally relevant failure modes for FPs, some of which will be
F1582 Terminology Relating to Spinal Implants
device-specific.Forexample,thesetestmethodsdonotaddress
implant wear resistance under expected in vivo loads and
3. Terminology
motions.Inaddition,thebiologicresponsetoweardebrisisnot
3.1 All functional and kinematic testing terminology is
addressed in these test methods.
consistent with the referenced standards (including Teminol-
1.5 Requirements are established for measuring displace-
ogy E6 and Terminology F1582), unless otherwise stated.
ments and evaluating the stiffness of an FP.
3.2 Definitions:
1.6 Some devices may not be testable in all test configura-
3.2.1 coordinate systems/axes—Global XYZorthogonalaxes
tions.
are defined following a right-handed Cartesian coordinate
1.7 The values stated in SI units are to be regarded as the system in which the XY plane is parallel to and co-planar with
standard with the exception of angular measurements, which the superior endplate of the inferior vertebral body.Alternative
may be reported in terms of either degrees or radians. coordinate systems may be used with justification. The global
axes are fixed relative to the inferior vertebral body. Lower
1.8 This standard does not purport to address all of the
case letters, xyz, denote a local moving orthogonal coordinate
safety concerns, if any, associated with its use. It is the
system attached to the superior vertebral body with directions
responsibility of the user of this standard to establish appro-
initially coincident with those of the global XYZ axes, respec-
tively. The 3D motion of the superior relative to inferior
ThispracticeisunderthejurisdictionofASTMCommitteeF04onMedicaland
Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.25 on Spinal Devices. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved Nov. 1, 2019. Published December 2019. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 2010. Last previous edition approved in 2014 as F2790-10(2014). DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/F2790-10R19E01. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
´1
F2790 − 10 (2019)
vertebra is specified and measured in terms of sequential
Eulerian angular rotations about the xyz axes, respectively (z
axial rotation, x lateral bend, and y flexion-extension).
3.2.1.1 origin—center of the global coordinate system that
is located at the posterior medial position on the superior
endplate of the inferior vertebral body.
3.2.1.2 X-axis—positive X-axis is directed anteriorly rela-
tive to the specimen’s initial unloaded position.
3.2.1.3 Y-axis—positive Y-axis is directed laterally (toward
the left) relative to the specimen’s initial unloaded position.
3.2.1.4 Z-axis—positive Z-axis is to be directed superiorly
relative to the specimen’s initial unloaded position.
3.2.2 failure—functional failure or substantial mechanical
failure.
3.2.2.1 functional failure—permanent deformation resulting
from fracture, plastic deformation, or loosening beyond the
ultimate displacement or loosening that renders the spinal
implant assembly ineffective or unable to adequately resist
load.
3.2.2.2 mechanical failure—failure associated with a defect
in the material (for example, fatigue crack) or of the bonding
between materials that may or may not produce functional
failure.
3.2.3 fatigue life—the number of cycles, N, that the FP can
sustain at a particular load or moment before failure occurs.
FIG. 1 UHMWPE Test Block
3.2.4 intended method of application—an FP may contain
different types of features to stabilize the implant-tissue inter-
face such as threads, spikes, and textured surfaces. Each type
of feature has an intended method of application or attachment
to the spine.
3.2.11 spinal implant assembly—a complete spinal implant
configuration as intended for surgical use. A spinal implant
3.2.5 insertion point of an anchor—the location where the
assembly may contain anchors, interconnections, longitudinal
anchor is attached to the test block.The insertion points shown
elements, and transverse elements.
inFig.1aretobeadheredtoifpossible.Insituationswherethe
design of the spinal implant assembly or the manufacturer’s
3.2.12 stiffness (axial—N/mm, angular—N·mm/degree or
surgical instructions for installation dictate otherwise, the
N·mm/radian)—the slope of the initial linear portion of the
attachment points may deviate from these dimensions.
load-displacement curve or the slope of the initial linear
portion of the moment-angular displacement curve. This is
3.2.6 longitudinal direction—the initial spatial orientation
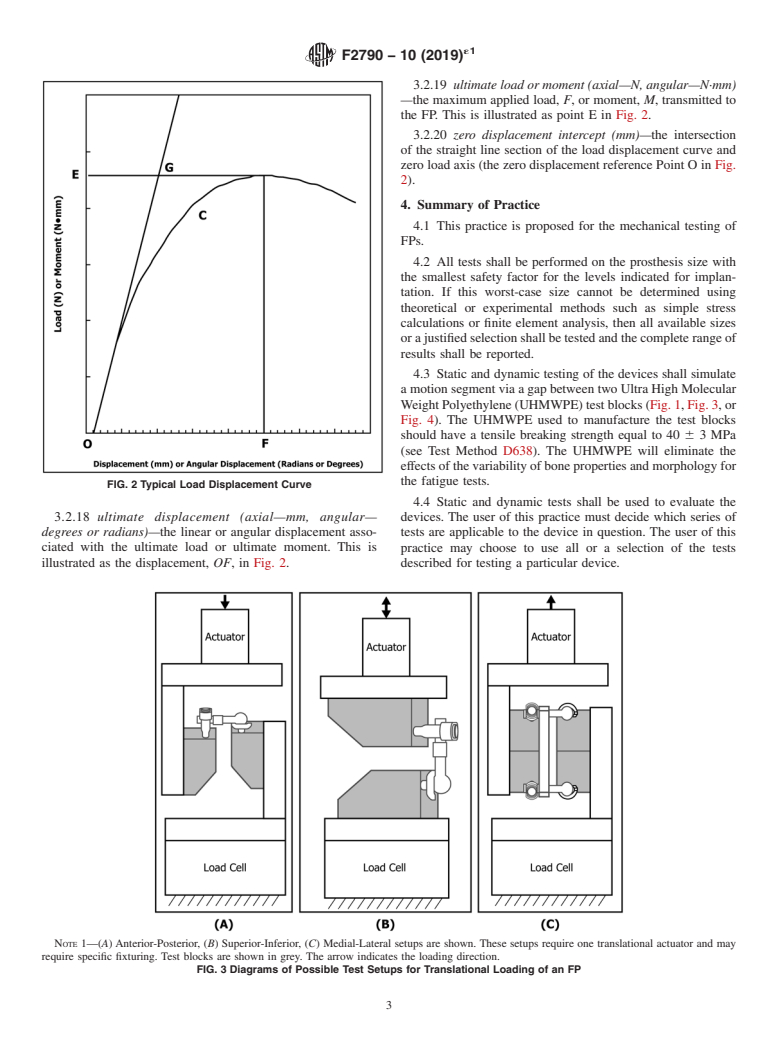
illustratedastheslopeoftheline OGinFig.2.Thedevicemay
between the insertion points in the superior test blocks and the
not exhibit an isolated linear portion on the load/displacement
inferior test blocks.
curve, due to the complicated nature of these devices.As such,
3.2.7 maximum run-out load or moment—the maximum
these data are for information only.
load or moment for a given test that can be applied to an FP
3.2.13 superior/inferior spinal implant construct—the supe-
where all of the tested constructs have withstood 10 000 000
rior or inferior spinal implant assembly attached to the test
cycles without failure.
block.
3.2.8 mechanical deterioration—deterioration that is visible
3.2.14 test block—the component of the test apparatus for
to the naked eye and is associated with mechanical damage to
mounting the FP in the intended test configuration.
thedeviceundertest(forexample,initiationoffatiguecrackor
surface wear). 3.2.15 tightening torque—the specified torque that is ap-
plied to the various fasteners of the spinal implant assembly.
3.2.9 permanent deformation—the remaining linear or an-
gular displacement (axial—mm, angular—degrees or radians) 3.2.16 torsional ultimate load (N·m)—the maximum torque
relative to the initial unloaded condition of the FP after the applied to a spinal implant assembly (the torque at Point E in
applied load or moment has been removed. Fig. 2). The ultimate torque should be a function of the device
and not of the load cell or testing machine.
3.2.10 radius of rotation—the distance between the center
of rotation and the functional position (for example, load- 3.2.17 total facet prosthesis—nonbiologic structure in-
bearing contact point) of the FP, for a given motion (that is, tended to restore the support and motion of the vertebral facet
flexion/extension, lateral bending, or axial rotation). joint.
´1
F2790 − 10 (2019)
3.2.19 ultimate load or moment (axial—N, angular—N·mm)
—the maximum applied load, F, or moment, M, transmitted to
the FP. This is illustrated as point E in Fig. 2.
3.2.20 zero displacement intercept (mm)—the intersection
of the straight line section of the load displacement curve and
zero load axis (the zero displacement reference Point O in Fig.
2).
4. Summary of Practice
4.1 This practice is proposed for the mechanical testing of
FPs.
4.2 All tests shall be performed on the prosthesis size with
the smallest safety factor for the levels indicated for implan-
tation. If this worst-case size cannot be determined using
theoretical or experimental methods such as simple stress
calculations or finite element analysis, then all available sizes
orajustifiedselectionshallbetestedandthecompleterangeof
results shall be reported.
4.3 Static and dynamic testing of the devices shall simulate
a motion segment via a gap between two Ultra High Molecular
Weight Polyethylene (UHMWPE) test blocks (Fig. 1, Fig. 3,or
Fig. 4). The UHMWPE used to manufacture the test blocks
should have a tensile breaking strength equal to 40 6 3 MPa
(see Test Method D638). The UHMWPE will eliminate the
effectsofthevariabilityofbonepropertiesandmorphologyfor
the fatigue tests.
FIG. 2 Typical Load Displacement Curve
4.4 Static and dynamic tests shall be used to evaluate the
3.2.18 ultimate displacement (axial—mm, angular— devices. The user of this practice must decide which series of
degrees or radians)—the linear or angular displacement asso- tests are applicable to the device in question. The user of this
ciated with the ultimate load or ultimate moment. This is practice may choose to use all or a selection of the tests
illustrated as the displacement, OF,in Fig. 2. described for testing a particular device.
NOTE 1—(A) Anterior-Posterior, (B) Superior-Inferior, (C) Medial-Lateral setups are shown. These setups require one translational actuator and may
require specific fixturing. Test blocks are shown in grey. The arrow indicates the loading direction.
FIG. 3 Diagrams of Possible Test Setups for Translational Loading of an FP
´1
F2790 − 10 (2019)
NOTE 1—(A) Simulated Flexion-Extension, (B)Axial Rotation, (C) Lateral Bending setups are shown.These setups require one rotational actuator and
may require specific fixturing. The arrow indicates the rotation direction. Test blocks are shown in grey. The position of the axis of rotation should be
based on the information in Table X1.1.
FIG. 4 Diagrams of Possible Test Setups for Rotational Loading of an FP
4.5 This practice is intended to be applicable to FPs that 2 Hz for non-metallic devices. Other test environments such as
support and transmit motion by means of an articulating joint a simulated body fluid, a saline drip or mist, distilled water,
or by use of compliant materials and/or design. Ceramics, other type of lubrication or dry could also be used with
metals, and/or polymers may be used in FPdesign, and it is the adequate justification. Likewise, alternative test frequencies
goal of this practice to enable a comparison of these devices, may be used with adequate justification to ensure that they do
regardless of material and type of device. not impact the device performance.
5.5 It is well known that the failure of materials is depen-
5. Significance and Use
dent upon stress, test frequency, surface treatments, and envi-
5.1 Facet Prosthesis Components—The facet replacement
ronmental factors. Therefore, when determining the effect of
may comprise a variety of shapes and configurations. Its forms
changing these parameters (for example, frequency, material,
may include, but are not limited to, ball and socket articulating
or environment), care should be taken to allow for appropriate
joints, joints having a free-floating or semi-constrained third
interpretation of the results. In particular, it may be necessary
body, metallic load-bearing surfaces, and spring and dampen-
to assess the influence of test frequency on device fracture
ing mechanisms. Additionally, it may have a unilateral or
while holding the test environment, implant materials and
bilateral design.
processing, and implant geometry constant.
5.2 These test methods are designed to quantify the static
and dynamic characteristics of different designs of FPs. The 6. Apparatus and Setup
tests are conducted in vitro in order to allow for analysis of
6.1 Test machines shall conform to the requirements of
individual devices and comparison of the mechanical perfor-
Practices E4.
mance of multiple designs.
6.2 The test apparatus shall allow multiple loading regimes
5.3 TheloadsappliedtotheFPmaydifferfromthecomplex
to be applied to all forms of FP.
loading seen in vivo, and therefore, the results from these tests
6.3 The test block should be created according to Fig. 1.
may not directly predict in vivo performance. The results,
Variations from this design to accommodate a device’s fixation
however, can be used to compare mechanical performance in
method or features should be reported and justified.
different devices.
5.4 Fatigue testing in a simulated body fluid or saline may 6.4 The interpedicular spacing (superior-to-inferior center-
cause fretting, corrosion, or lubricate the interconnections and to-center distance between bone anchors) shall be set at 38 mm
thereby affect the relative performance of tested devices. This when installing the device and at the beginning of each test.
test should be conducted in a 0.9 % salin
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.