ASTM D7493-08
(Test Method)Standard Test Method for Online Measurement of Sulfur Compounds in Natural Gas and Gaseous Fuels by Gas Chromatograph and Electrochemical Detection
Standard Test Method for Online Measurement of Sulfur Compounds in Natural Gas and Gaseous Fuels by Gas Chromatograph and Electrochemical Detection
SIGNIFICANCE AND USE
Gaseous fuels, such as natural gas, petroleum gases and bio-gases, contain varying amounts and types of sulfur compounds. These sulfur compounds are generally odorous, corrosive to equipment, and can inhibit or destroy catalysts employed in gas processing and end use, such as those used in fuel cell. Their accurate on-line measurement is essential to gas processing, operation and utilization, and of regulatory interest.
Small amounts (typically, total 4-6 PPMv) of sulfur odorants are added to natural gas and other fuel gases for safety purposes. Some sulfur odorants can be reactive, and may be oxidized, forming more stable sulfur compounds having lower odor thresholds. These gaseous fuels are analyzed for sulfur odorants to help in monitoring and to ensure appropriate odorant levels for public safety.
This method offers an on-line technique to continuously identify and quantify individual target sulfur species in gaseous fuel with automatic calibration and validation.
SCOPE
1.1 This test method is for on-line measurement of volatile sulfur-containing compounds in gaseous fuels by gas chromatography (GC) and electrochemical (EC) detection. The test method is applicable to hydrogen sulfide, C1-C4 mercaptans, sulfides and tetrahydrothiophene (THT).
1.1.1 Carbonyl sulfide (COS) is not covered in this test method.
1.1.2 The detection range for sulfur compounds is approximately from 0.1 to 100 PPMv or 0.1 to 100 mg/m3. The detection range may vary depending on the sample injection volume, chromatographic peak separation and the sensitivity of specific EC detector.
1.2 This test method describes a GC-EC method employing packed GC columns and a specific detector as an illustration for natural gas and other gaseous fuel containing mainly light hydrocarbons. Alternative GC columns, detector designs and instrument parameters may be used for the same analysis or for different types of gaseous fuel, provided that appropriate chromatographic separation and optimal detection of these compounds can be achieved.
1.3 This test method does not intend to identify and measure all individual sulfur species, and is mainly employed for monitoring natural sulfur and sulfur odorant compounds commonly found in fuel gases or employed as an odorous warning agent in fuel gases.
1.4 The test method is normally employed for repetitive on-line monitoring of sulfur components in fuel gases with a single sulfur standard. The test method may be employed for laboratory-quality measurement with more extensive calibration. (See Test Methods D 5504, D 5623, D 6228, D 6968, ISO 19739, ISO 6326-2, and GPA 2199.)
1.5 The test method can be used for measurement of all listed sulfur compounds in air or other gases, provided that no compound, which can interfere with the GC separation and electrochemical detection, is present.
1.6 This test method is written in conjunction with Practices D 5287, D 7165 and D 7166.
1.7 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D7493 − 08
StandardTest Method for
Online Measurement of Sulfur Compounds in Natural Gas
and Gaseous Fuels by Gas Chromatograph and
Electrochemical Detection
This standard is issued under the fixed designation D7493; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope compound, which can interfere with the GC separation and
electrochemical detection, is present.
1.1 This test method is for on-line measurement of volatile
sulfur-containing compounds in gaseous fuels by gas chroma- 1.6 This test method is written in conjunction with Practices
tography (GC) and electrochemical (EC) detection. The test D5287, D7165 and D7166.
method is applicable to hydrogen sulfide, C1-C4 mercaptans,
1.7 The values stated in SI units are to be regarded as
sulfides and tetrahydrothiophene (THT).
standard. No other units of measurement are included in this
1.1.1 Carbonyl sulfide (COS) is not covered in this test
standard.
method.
1.8 This standard does not purport to address all of the
1.1.2 The detection range for sulfur compounds is approxi-
safety concerns, if any, associated with its use. It is the
mately from 0.1 to 100 PPMv or 0.1 to 100 mg/m . The
responsibility of the user of this standard to establish appro-
detection range may vary depending on the sample injection
priate safety and health practices and determine the applica-
volume,chromatographicpeakseparationandthesensitivityof
bility of regulatory limitations prior to use.
specific EC detector.
1.2 This test method describes a GC-EC method employing 2. Referenced Documents
packed GC columns and a specific detector as an illustration
2.1 ASTM Standards:
for natural gas and other gaseous fuel containing mainly light
D3609 Practice for Calibration Techniques Using Perme-
hydrocarbons. Alternative GC columns, detector designs and
ation Tubes
instrumentparametersmaybeusedforthesameanalysisorfor
D4150 Terminology Relating to Gaseous Fuels
different types of gaseous fuel, provided that appropriate
D4626 Practice for Calculation of Gas Chromatographic
chromatographic separation and optimal detection of these
Response Factors
compounds can be achieved.
D5287 Practice for Automatic Sampling of Gaseous Fuels
1.3 Thistestmethoddoesnotintendtoidentifyandmeasure D5504 TestMethodforDeterminationofSulfurCompounds
all individual sulfur species, and is mainly employed for in Natural Gas and Gaseous Fuels by Gas Chromatogra-
phy and Chemiluminescence
monitoring natural sulfur and sulfur odorant compounds com-
monly found in fuel gases or employed as an odorous warning D5623 Test Method for Sulfur Compounds in Light Petro-
leum Liquids by Gas Chromatography and Sulfur Selec-
agent in fuel gases.
tive Detection
1.4 The test method is normally employed for repetitive
D6228 TestMethodforDeterminationofSulfurCompounds
on-line monitoring of sulfur components in fuel gases with a
in Natural Gas and Gaseous Fuels by Gas Chromatogra-
single sulfur standard. The test method may be employed for
phy and Flame Photometric Detection
laboratory-quality measurement with more extensive calibra-
D6968 Test Method for Simultaneous Measurement of Sul-
tion. (See Test Methods D5504, D5623, D6228, D6968, ISO
fur Compounds and Minor Hydrocarbons in Natural Gas
19739, ISO 6326-2, and GPA 2199.
and Gaseous Fuels by Gas Chromatography and Atomic
1.5 The test method can be used for measurement of all
Emission Detection
listed sulfur compounds in air or other gases, provided that no
D7165 Practice for Gas Chromatograph Based On-line/At-
line Analysis for Sulfur Content of Gaseous Fuels
ThistestmethodisunderthejurisdictionofASTMCommitteeD03onGaseous
Fuels and is the direct responsibility of Subcommittee D03.12 on On-Line/At-Line For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Analysis of Gaseous Fuels. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Current edition approved Dec. 15, 2008. Published January 2009. DOI: 10.1520/ Standards volume information, refer to the standard’s Document Summary page on
D7493-08. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D7493 − 08
D7166 Practice for Total SulfurAnalyzer Based On-line/At- rosive to equipment, and can inhibit or destroy catalysts
line for Sulfur Content of Gaseous Fuels employed in gas processing and end use, such as those used in
fuelcell.Theiraccurateon-linemeasurementisessentialtogas
2.2 ISO Standards:
processing,operationandutilization,andofregulatoryinterest.
ISO 19739 Natural Gas – Determination of Sulfur Com-
pounds by Gas chromatography
5.2 Small amounts (typically, total 4-6 PPMv) of sulfur
ISO 6326-2 GasAnalysis – Determination of Sulphur Com-
odorantsareaddedtonaturalgasandotherfuelgasesforsafety
pounds in Natural gas – Part 2: Gas Chromatographic
purposes. Some sulfur odorants can be reactive, and may be
Method Using an Electrochemical Detector for The De-
oxidized, forming more stable sulfur compounds having lower
termination of Odoriferous Sulphur Compounds
odor thresholds. These gaseous fuels are analyzed for sulfur
2.3 GPA Standard
odorants to help in monitoring and to ensure appropriate
GPA2199 Determination - Determination of Specific Sulfur
odorant levels for public safety.
Compounds by Capillary Gas Chromatography and Sulfur
5.3 This method offers an on-line technique to continuously
Chemiluminescence Detection
identifyandquantifyindividualtargetsulfurspeciesingaseous
fuel with automatic calibration and validation.
3. Terminology
3.1 Common terminology used in this method are cited in
6. Apparatus
Terminology D4150 3.2 Sulfur compounds are commonly
6.1 Chromatograph—Industrial gas chromatograph with
referred by their initials (chemical or formula), for example,
isothermal oven and automatic injection valve and software
3.2 Abbriviations:
necessary for interfacing to a chromic acid electrochemical
detector and for the intended application and performance.The
hydrogen sulfide =H S
GC system must be inert, well conditioned and passivated with
methyl mercaptan = MeSH (MM)
a gas containing the sulfur compounds of interest to ensure
ethyl mercaptan = EtSH (EM)
reliable results.
dimethyl sulfide = DMS
6.1.1 Sample Inlet System—Thegassampleisintroducedby
i-Propyl mercaptan = IPM
sample loop injection. An automated non-reactive gas sam-
n-Propyl mercaptan = NPM
pling valve is employed for fixed sample loop injection. The
t-Butyl mercaptan = TBM
sample injection port must be heated continuously at a tem-
tetrahydrothiophene = THT or Thiophane
perature significantly (~10°C) above the temperature at which
4. Summary of Test Method the gas was sampled to avoid sample condensation and
discrimination. Inert tubing made of non-permeable, non-
4.1 Gaseous fuel is directly sampled on-line for analysis of
sorbing and non-reactive materials, as short as possible and
specific and normally reactive sulfur compounds. Samples are
heat traced at the same temperature, should be employed for
introducedtoaninertGCinstrumentthroughaninertsampling
transferring the sample from a sample source to the gas
system. Sulfur compounds are separated by a GC column and
sampling valve and to the GC inlet system. Silica-coated 316
measured by an EC detector. The method requires frequent
stainless steel (s.s.) and non-permeable Teflon type tubing are
calibrationusingstablestandards.Thetestmethodconformsto
often employed. Different size fixed-volume sampling loops
the standard practice of Practice D7165.
(0.25 to 10.0 mL) may be used for target concentration ranges,
4.2 A fixed volume of the fuel gas (normally 0.25 mL) is
provided with adequate chromatographic separation. The same
injected into an isothermal gas chromatograph where it is
non-reactive materials are used for the sampling loop to avoid
passed through a 1.2 meter, 1.6 mm I.D., Chromosorb W
possible decomposition or absorption of reactive species.
column. A varying amount of sample and other GC columns
When necessary, a precision glass syringe with a gas-tight
with or without column back-flush technique can be used for
Teflon-seated plunger is used to manually introduce sample or
sensitive detection of sulfur with optimal separation.
calibrationstandardsthroughaPTFEseptumatthefrontofGC
4.3 Specific GC-separated sulfur compounds are detected columns. The sampling and GC inlet system must be well
by an electrochemical detector utilizing chromic acid electro- conditioned and evaluated frequently for compatibility with
lyte. Detectors with different physical designs are commer- tracequantitiesofreactivesulfurcompounds,suchastert-butyl
cially available and may be employed. mercaptan. A programmable and computer-controlled multi-
stream sample selector can be used to sample fuel gases and
5. Significance and Use
calibration gases.
6.1.2 Column Temperature—The gas chromatograph must
5.1 Gaseous fuels, such as natural gas, petroleum gases and
be capable of maintaining an isothermal temperature, normally
bio-gases, contain varying amounts and types of sulfur com-
at 65 °C, with temperature variation not exceeding 0.5 °C
pounds. These sulfur compounds are generally odorous, cor-
6.1.3 Carrier and Detector Gas Control—Constant flow
control of carrier and detector gases is necessary for optimum
Available from International Organization for Standardization (ISO), 1, ch. de
andconsistentanalyticalperformance.Controlisbestprovided
la Voie-Creuse, Case postale 56, CH-1211, Geneva 20, Switzerland, http://
by the use of pressure regulators and fixed flow restrictors.The
www.iso.ch.
gas flow rate is measured by any appropriate means and the
Available from Gas ProcessorsAssociation (GPA), 6526 E. 60th St.,Tulsa, OK
74145, http://www.gasprocessors.com. required gas flow indicated by the use of a pressure gauge.
D7493 − 08
Mass flow controllers, capable of maintaining gas flow con- current which is measured in a low resistance measuring
stant to 6 1 % at the required flow rates can also be used. The circuit. For example, to t-butyl mercaptan is oxidized t-butyl
supply pressure of the gas delivered to the gas chromatograph
sulfoxide and chromium oxide (Eq 2)
must be at least 69 kPa (10 psig) greater than the regulated gas
2 CrO 12 R 2 SH→2 RS 5 O1Cr O (1)
3 2 3
at the instrument to compensate for the system back pressure.
where:
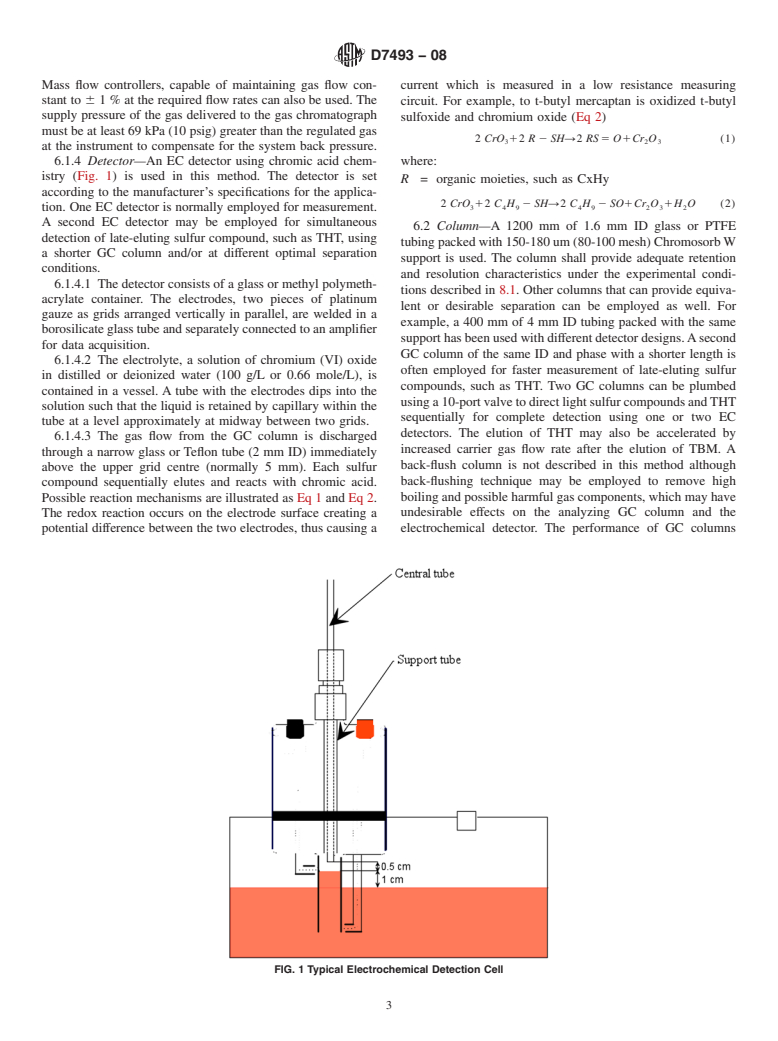
6.1.4 Detector—An EC detector using chromic acid chem-
istry (Fig. 1) is used in this method. The detector is set
R = organic moieties, such as CxHy
according to the manufacturer’s specifications for the applica-
2 CrO 12 C H 2 SH→2 C H 2 SO1Cr O 1H O (2)
3 4 9 4 9 2 3 2
tion. One EC detector is normally employed for measurement.
A second EC detector may be employed for simultaneous
6.2 Column—A 1200 mm of 1.6 mm ID glass or PTFE
detection of late-eluting sulfur compound, such as THT, using
tubingpackedwith150-180um(80-100mesh)ChromosorbW
a shorter GC column and/or at different optimal separation
support is used. The column shall provide adequate retention
conditions.
and resolution characteristics under the experimental condi-
6.1.4.1 The detector consists of a glass or methyl polymeth-
tions described in 8.1. Other columns that can provide equiva-
acrylate container. The electrodes, two pieces of platinum
lent or desirable separation can be employed as well. For
gauze as grids arranged vertically in parallel, are welded in a
example, a 400 mm of 4 mm ID tubing packed with the same
borosilicate glass tube and separately connected to an amplifier
supporthasbeenusedwithdifferentdetectordesigns.Asecond
for data acquisition.
GC column of the same ID and phase with a shorter length is
6.1.4.2 The electrolyte, a solution of chromium (VI) oxide
often employed for faster measurement of late-eluting sulfur
in distilled or deionized water (100 g/L or 0.66 mole/L), is
compounds, such as THT. Two GC columns can be plumbed
contained in a vessel. A tube with the electrodes dips into the
usinga10-portvalvetodirectlightsulfurcompoundsandTHT
solution such that the liquid is retained by capillary within the
sequentially for complete detection using one or two EC
tube at a level approximately at midway between two grids.
detectors. The elution of THT may also be accelerated by
6.1.4.3 The gas flow from the GC column is discharged
increased carrier gas flow rate after the elution of TBM. A
through a narrow glass or Teflon tube (2 mm ID) immediately
back-flush column is not described in this method although
above the upper grid centre (normally 5 mm). Each sulfur
back-flushing technique may be employed to remove high
compound sequentially elutes and reacts with chromic acid.
boiling and possible harmful gas components, which may have
Possible reaction mechanisms are illustrated as Eq 1 and Eq 2.
undesirable effects on the analyzing GC column and the
The redox reaction occurs on the electrode surface creating a
potential difference between the two electrodes, thus causing a electrochemical detector. The performance of GC columns
FIG. 1 Typical Electrochemical Detection Cell
D7493 − 08
shall give adequate separation of target sulfur compounds for other suitable agents to remove hydrocarbons or oxygen, or
desired accuracy and precision. both, in helium and nitrogen. Available carrier gas pressure
must be sufficient to ensure a constant carrier gas flow rate (see
6.3 Data Acquisition
6.1.4).
6.3.1 The device and software must have the following
capabilities:
NOTE 2—Warning: Carrier gas employed may be from a source of
compressed gases under high pressure.
6.3.1.1 Graphic presentation of the chromatogram.
6.3.1.2 Digital display of chromatographic peak areas.
7.4 Chromium Oxide—Reagent grade (99.9% minimum pu-
6.3.1.3 Identification of peaks by retention time or relative
rity).
retention time, or both.
NOTE 3—Warning: Toxic chemical, handling with rubber gloves and
6.3.1.4 Calculation and use of response factors.
caution. Waste reagent should be chemically reduced and properly
6.3.1.5 External standard calculation and data presentation.
disposed.
6.3.1.6 Instrument control for ED operation, such as gas
8. Preparation of Apparatus and Calibration
pressure and flow control.
8.1 Chromatograph—Place in service according to the
7. Reagents and Materials
manufacturer’s instructions. Typical operating conditions are
shown in Table 1.
7.1 Compressed Cylinder Gas Standards—Gas standards
should be of high purity with certified stabili
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.