ASTM F2059-06(2012)e1
(Test Method)Standard Test Method for Laboratory Oil Spill Dispersant Effectiveness Using The Swirling Flask
Standard Test Method for Laboratory Oil Spill Dispersant Effectiveness Using The Swirling Flask
SIGNIFICANCE AND USE
A standard test is necessary to establish a baseline performance parameter so that dispersants can be compared, a given dispersant can be compared for effectiveness on different oils, and at different oil weathering stages, and batches of dispersant or oils can be checked for effectiveness changes with time or other factors.
Dispersant effectiveness varies with oil type, sea energy, oil conditions, salinity, and many other factors. Test results from this test method form a baseline, but are not to be taken as the absolute measure of performance at sea. Actual field effectiveness could be more or less than this value.
Many dispersant tests have been developed around the world. This test has been developed over many years using findings from world-wide testing to use standardized equipment, test procedures, and to overcome difficulties noted in other test procedures.
SCOPE
1.1 This test method covers the procedure to determine the effectiveness of oil spill dispersants on various oils in the laboratory. This test method is not applicable to other chemical agents nor to the use of such products or dispersants in open waters.
1.2 This test method covers the use of the swirling flask test apparatus and does not cover other apparatuses nor are the analytical procedures described in this report directly applicable to such procedures.
1.3 The test results obtained using this test method are intended to provide baseline effectiveness values used to compare dispersants and oil types under conditions analogous to those used in the test.
1.4 The test results obtained using this test method are effectiveness values that should be cited as test values derived from this standard test. Dispersant effectiveness values do not directly relate to effectiveness at sea or in other apparatuses. Actual effectiveness at sea is dependant on sea energy, oil state, temperature, salinity, actual dispersant dosage, and amount of dispersant that enters the oil.
1.5 The decision to use or not use a dispersant on an oil should not be based solely on this or any other laboratory test method.
1.6 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.7 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
´1
Designation: F2059 − 06 (Reapproved 2012)
Standard Test Method for
Laboratory Oil Spill Dispersant Effectiveness Using the
Swirling Flask
This standard is issued under the fixed designation F2059; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
ε NOTE—An editorial change was made to Section 6 in February 2012.
1. Scope 2. Summary of Test Method
1.1 This test method covers the procedure to determine the 2.1 Dispersant is pre-mixed with oil and placed on water in
effectiveness of oil spill dispersants on various oils in the a test vessel. The test vessel is agitated on a moving table
laboratory.This test method is not applicable to other chemical shaker. At the end of the shaking period, a settling period is
agents nor to the use of such products or dispersants in open specified and then a sample of water taken.The oil in the water
waters. column is extracted from the water using a pentane/
dichloromethane mixture and analyzed using gas chromatog-
1.2 This test method covers the use of the swirling flask test
raphy.
apparatus and does not cover other apparatuses nor are the
analytical procedures described in this report directly appli- 2.2 The extract is analyzed for oil using a gas chromato-
cable to such procedures. graph equipped with a flame ionization detector, (GC-FID).
Quantification is by means of the internal standard method.
1.3 The test results obtained using this test method are
Effectiveness values are derived by comparison with a cali-
intended to provide baseline effectiveness values used to
brated set of effectiveness values obtained at the same time and
compare dispersants and oil types under conditions analogous
by the same method.
to those used in the test.
3. Significance and Use
1.4 The test results obtained using this test method are
effectiveness values that should be cited as test values derived
3.1 A standard test is necessary to establish a baseline
from this standard test. Dispersant effectiveness values do not
performance parameter so that dispersants can be compared, a
directly relate to effectiveness at sea or in other apparatuses.
given dispersant can be compared for effectiveness on different
Actualeffectivenessatseaisdependantonseaenergy,oilstate,
oils, and at different oil weathering stages, and batches of
temperature, salinity, actual dispersant dosage, and amount of
dispersant or oils can be checked for effectiveness changes
dispersant that enters the oil.
with time or other factors.
1.5 The decision to use or not use a dispersant on an oil
3.2 Dispersant effectiveness varies with oil type, sea energy,
should not be based solely on this or any other laboratory test
oil conditions, salinity, and many other factors. Test results
method.
from this test method form a baseline, but are not to be taken
as the absolute measure of performance at sea. Actual field
1.6 The values stated in SI units are to be regarded as
effectiveness could be more or less than this value.
standard. No other units of measurement are included in this
standard.
3.3 Many dispersant tests have been developed around the
world. This test has been developed over many years using
1.7 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the findings from world-wide testing to use standardized
equipment, test procedures, and to overcome difficulties noted
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- in other test procedures.
bility of regulatory limitations prior to use.
4. Interferences and Sources of Error
4.1 Interferences can be caused by contaminants, particu-
larly residual oil or surfactants in solvents, on glassware, and
This test method is under the jurisdiction of ASTM Committee F20 on
Hazardous Substances and Oil Spill Response and is the direct responsibility of
other sample processing apparatus that lead to discrete artifacts
Subcommittee F20.13 on Treatment.
or elevated baselines in gas chromatograms. All glassware
Current edition approved Feb. 1, 2012. Published February 2012. Originally
must be thoroughly cleaned. The cleaning process includes
approved in 2000. Last previous edition approved in 2006 as F2059 – 06. DOI:
10.1520/F2059-06R12E01. rinsing with dichloromethane to remove the oil, followed by
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
´1
F2059 − 06 (2012)
rinsing three times each with tap water, purified water (reverse 4.11 Extreme care should be taken when applying the oil to
osmosis), and acetone. Once cleaned, precautions must be thesurfacesothatmixingdoesnotoccur.Theoilshouldgently
taken to minimize contact of the glassware with surfactants to glide across the water to form a slick. If the oil streams out into
prevent undesired interferences. the water, the agitation can disperse the oil, increasing the
amount of oil dispersed and erroneously raising the final
4.2 Dispersant effectiveness is very susceptible to energy
dispersion result.
levels. Table top shakers generally start and stop slowly.
Shakers that start motion rapidly and stop suddenly impart a 4.12 A slick may form at the water surface in the spout of
high energy to the system and thus cause more dispersion than the swirling flask during mixing and settling. It is important
would be the case with a normal shaker. Furthermore, this thisoildoesnotenterthewatersampledforanalysis.Therefore
variation would not be repeatable. The shaker table used it is important to drain the contents of the spout (about 3 mL)
shouldbeobservedforrapidmovementsorstopstoensurethat prior to sampling, and ensure any adhering droplets do not
it is usable for these tests. The rotational speed of the shaker enter the sample.
should be checked with a tachometer every week.
4.13 The procedure is time critical for the elements of
4.3 The Erlenmeyer flasks used in this test are tapered and mixing, settling, and sampling. Care should be taken to adhere
the energy level varies with the amount of fill. to the times indicated in the procedure for both the mixing and
settling element, as variations in energy input, and especially
4.4 The output is highly sensitive to the volume of oil,
time allowed for droplet creaming, can impact results. Since
water, and extractant delivered. All pipets and dispensers
the water samples cannot be sampled simultaneously, this step
should be calibrated frequently and verified daily.
must be performed with as much careful haste as possible, to
4.5 Theuseofpositivedisplacementpipetsismandatoryfor
reduce the difference in settling times experienced by the
all controlled volumes of microlitre quantities. Use of volume
samples in the test run.
displacement pipets will result in erroneous results due to the
4.14 Analysis of the gas chromatograph-detectable Total
viscosity of the dispersants and oils, the variable viscosity of
Petroleum Hydrocarbon (TPH) content is subject to variability
the oils to be tested (some semi-solid), and the density of
in GC operation and repeatability. Therefore, it is imperative
dichloromethane.
that a rigorous quality assurance program is in place to ensure
4.6 The order of addition of the dispersant and oil has
the GC is functioning properly and valid results are obtained.
effectsontheaccuracyofresults,asthedispersantmayinteract
4.15 Care should be taken to determine the baseline in a
with the vessel walls if added first, thereby reducing the
valid and repeatable manner for both samples and calibration
quantityavailableinthepremix.Itisthereforeimportanttoadd
sets.
oil to the vessel first, and add the dispersant directly to the oil.
Asecondadditionofoilissuggestedsimplybecauseitiseasier 4.16 The accuracy and repeatability of the test can be
to control a large volume of oil than a minute volume of verified and compared using standard oil and dispersant
dispersant when attempting to achieve a specific ratio of 25:1. samples
4.7 Following surfactant addition, vigorous mixing is re-
5. Apparatus
quired to thoroughly homogenize the sample. Sharp, manual
strokes are suggested for light oils, while very heavy oils may 5.1 Modified 120-mL Erlenmeyer Flask, used as the test
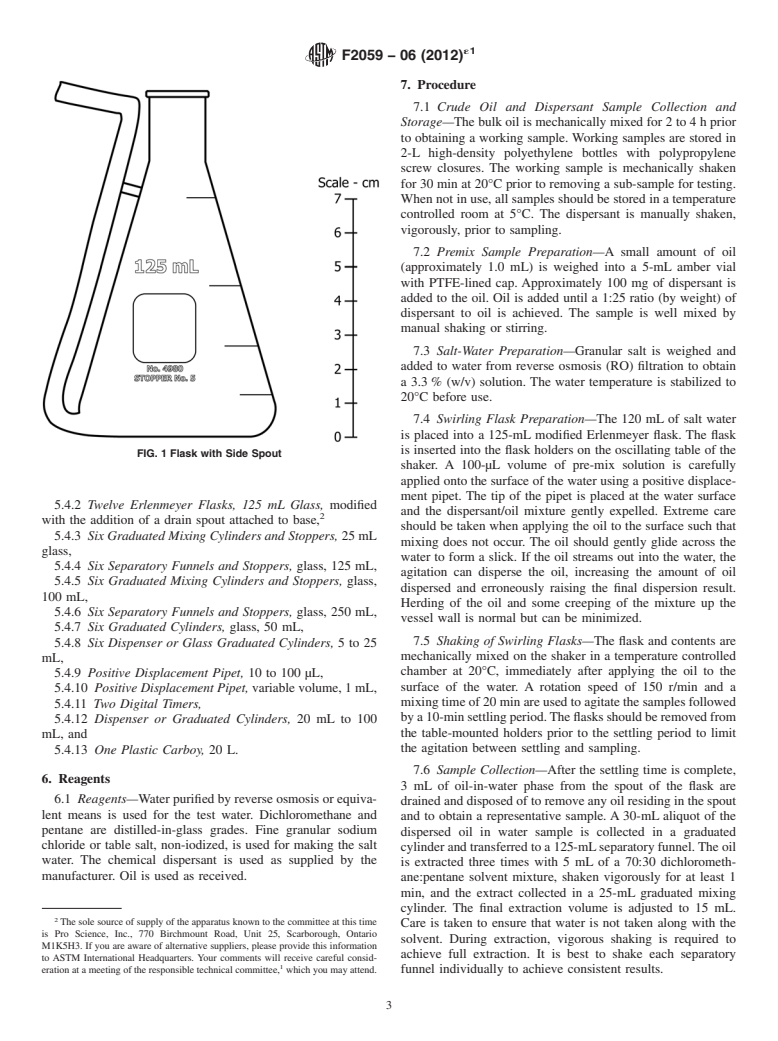
require stirring with a clean glass rod or spatula. vessel.Aside spout is added to a standard Erlenmeyer flask to
enable sampling from the water column with minimal distur-
4.8 There are indications that the results for some premixed
bance of resurfaced oil. This vessel is illustrated in Fig. 1.
dispersant/oilcombinationschangeovertime.Itisnecessaryto
take precautions against this potential source of variation. The 5.2 Moving-Table Shaker, with an orbital motion of 1 in.
testing should be concluded as soon as possible after the (25.4 mm) and fitted with flask holders. Ideally such shakers
premix is prepared, generally within a few hours. Results from should be constructed inside temperature-controlled chambers.
samples stored for periods as long as a week should not be Ifsuchanenclosedchamberisnotused,themeasurementmust
considered reliable. be conducted inside temperature-controlled rooms.
4.9 Since the performance of the dispersant is affected by 5.3 Gas Chromatograph (GC), equipped with a flame ion-
salinity, thorough mixing of the salt water is required. Care ization detector is used for analysis. The column is a fused
should also be observed to avoid evaporation from open silica column.
containers of salt water. Over a period of days and weeks, the
5.4 The following is a list of other necessary supplies.
loss of water can significantly increase the salinity.An airtight
Suppliers of suitable units are footnoted. Equivalent supplies
closure is recommended to maintain salinity levels at 3.3 %.
areacceptableineverycase.Quantitiesofsuppliesaregivento
conduct a full set of six samples and calibration set:
4.10 Temperature is a factor in dispersion, so it is important
that all components (salt water, pre-mix, and temperature 5.4.1 Eighteen Crimp Style Vials, with aluminum/PTFE
controlled chamber) are stable at 20°C before starting. (polytetrafluoroethylene) seals, 12 by 32 mm,
´1
F2059 − 06 (2012)
7. Procedure
7.1 Crude Oil and Dispersant Sample Collection and
Storage—The bulk oil is mechanically mixed for 2 to 4 h prior
to obtaining a working sample. Working samples are stored in
2-L high-density polyethylene bottles with polypropylene
screw closures. The working sample is mechanically shaken
for 30 min at 20°C prior to removing a sub-sample for testing.
When not in use, all samples should be stored in a temperature
controlled room at 5°C. The dispersant is manually shaken,
vigorously, prior to sampling.
7.2 Premix Sample Preparation—A small amount of oil
(approximately 1.0 mL) is weighed into a 5-mL amber vial
with PTFE-lined cap. Approximately 100 mg of dispersant is
added to the oil. Oil is added until a 1:25 ratio (by weight) of
dispersant to oil is achieved. The sample is well mixed by
manual shaking or stirring.
7.3 Salt-Water Preparation—Granular salt is weighed and
added to water from reverse osmosis (RO) filtration to obtain
a 3.3 % (w/v) solution. The water temperature is stabilized to
20°C before use.
7.4 Swirling Flask Preparation—The 120 mL of salt water
is placed into a 125-mL modified Erlenmeyer flask. The flask
is inserted into the flask holders on the oscillating table of the
FIG. 1 Flask with Side Spout
shaker. A 100-µL volume of pre-mix solution is carefully
applied onto the surface of the water using a positive displace-
ment pipet. The tip of the pipet is placed at the water surface
5.4.2 Twelve Erlenmeyer Flasks, 125 mL Glass, modified
and the dispersant/oil mixture gently expelled. Extreme care
with the addition of a drain spout attached to base,
should be taken when applying the oil to the surface such that
5.4.3 Six Graduated Mixing Cylinders and Stoppers, 25 mL
mixing does not occur. The oil should gently glide across the
glass,
water to form a slick. If the oil streams out into the water, the
5.4.4 Six Separatory Funnels and Stoppers, glass, 125 mL,
agitation can disperse the oil, increasing the amount of oil
5.4.5 Six Graduated Mixing Cylinders and Stoppers, glass,
dispersed and erroneously raising the final dispersion result.
100 mL,
Herding of the oil and some creeping of the mixture up the
5.4.6 Six Separatory Funnels and Stoppers, glass, 250 mL,
vessel wall is normal but can be minimized.
5.4.7 Six Graduated Cylinders, glass, 50 mL,
7.5 Shaking of Swirling Flasks—The flask and contents are
5.4.8 Six Dispenser or Glass Graduated Cylinders,5to25
mechanically mixed on the shaker in a temperature controlled
mL,
chamber at 20°C, immediately after applying the oil to the
5.4.9 Positive Displacement Pipet, 10 to 100 µL,
surface of the water. A rotation speed of 150 r/min and a
5.4.10 Positive Displacement Pipet, variable volume, 1 mL,
mixing time of 20 min are used to agitate the samples followed
5.4.11 Two Digital Timers,
bya10-minsettlingperiod.Theflasksshouldberemovedfrom
5.4.12 Dispenser or Graduated Cylinders, 20 mL to 100
the table-mounted holders prior to the settling period to limit
mL, and
the agitation between settling and sampling.
5.4.13 One Plastic Carboy, 20 L.
7.6 Sample Collection—After the settling time is complete,
6. Reagents
3 mL of oil-in-water phase from the spout of the flask are
6.1 Reagents—Water purified by reverse osmosis or equiva-
drained and disposed of to remove any oil residing in the spout
lent means is used for the test water. Dichloromethane and
and to obtain a representative sample. A 30-mL aliquot of the
pentane are distilled-in-glass grades. Fine granular sodium
dispersed oil in water sample is collected in a graduated
chloride or table salt, non-iodized, is used for making the salt
cylinder and transferred to a 125-mLseparatory funnel.The oil
water. The chemical dispersant is used as supplied by the
is extracted three times with 5 mL of a 70:30 dichlorometh-
manufacturer. Oil is used as received.
ane:pentane solvent mixture, shaken vigorously for at least 1
min, and the extract collected in a 25-mL graduated mixing
cylinder. The final extraction volume is adjusted to 15 mL.
The sole source of supply of the apparatus known to the committee at this time
Care is taken to ensure that water is not taken along with the
is Pro Science, Inc., 770 Birchmount Road, Unit 25, Scarborough, Ontario
solvent. During extraction, vigorous shaking is required to
M1K5H3. If you are aware of alter
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.