ASTM D1142-95(2012)
(Test Method)Standard Test Method for Water Vapor Content of Gaseous Fuels by Measurement of Dew-Point Temperature
Standard Test Method for Water Vapor Content of Gaseous Fuels by<brk/> Measurement of Dew-Point Temperature
SIGNIFICANCE AND USE
3.1 Generally, contracts governing the pipeline transmission of natural gas contain specifications limiting the maximum concentration of water vapor allowed. Excess water vapor can cause corrosive conditions, degrading pipelines and equipment. It can also condense and freeze or form methane hydrates causing blockages. Water–vapor content also affects the heating value of natural gas, thus influencing the quality of the gas. This test method permits the determination of water content of natural gas.
SCOPE
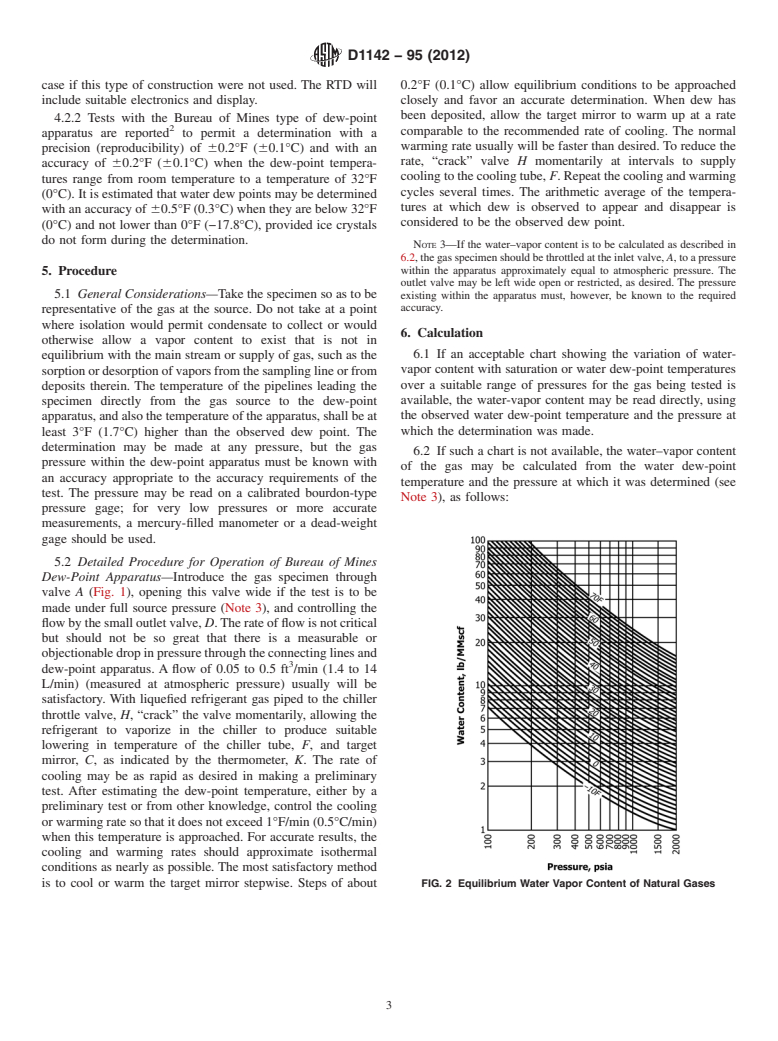
1.1 This test method covers the determination of the water vapor content of gaseous fuels by measurement of the dew-point temperature and the calculation therefrom of the water vapor content. Note 1—Some gaseous fuels contain vapors of hydrocarbons or other components that easily condense into liquid and sometimes interfere with or mask the water dew point. When this occurs, it is sometimes very helpful to supplement the apparatus in Fig. 1 with an optical attachment that uniformly illuminates the dew–point mirror and also magnifies the condensate on the mirror. With this attachment it is possible, in some cases, to observe separate condensation points of water vapor, hydrocarbons, and glycolamines as well as ice points. However, if the dew point of the condensable hydrocarbons is higher than the water vapor dew point, when such hydrocarbons are present in large amounts, they may flood the mirror and obscure or wash off the water dew point. Best results in distinguishing multiple component dew points are obtained when they are not too closely spaced.
FIG. 1 Bureau of Mines Dew-Point Apparatus
Note 2—Condensation of water vapor on the dew-point mirror may appear as liquid water at temperatures as low as 0 to −10°F (−18 to −23°C). At lower temperatures an ice point rather than a water dew point likely will be observed. The minimum dew point of any vapor that can be observed is limited by the mechanical parts of the equipment. Mirror temperatures as low as −150°F (−100°C) have been measured, using liquid nitrogen as the coolant with a thermocouple attached to the mirror, instead of a thermometer well.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D1142 − 95 (Reapproved 2012)
Standard Test Method for
Water Vapor Content of Gaseous Fuels by
1
Measurement of Dew-Point Temperature
This standard is issued under the fixed designation D1142; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope saturated with the gas mixture. When a gas containing water
vapor is at the water dew-point temperature, it is said to be
1.1 This test method covers the determination of the water
saturated at the existing pressure.
vapor content of gaseous fuels by measurement of the dew-
point temperature and the calculation therefrom of the water 2.1.2 specific volume—of a gaseous fuel, the volume of the
gas in cubic feet per pound.
vapor content.
2.1.3 water dew-point temperature— of a gaseous fuel, the
NOTE 1—Some gaseous fuels contain vapors of hydrocarbons or other
temperature at which the gas is saturated with water vapor at
components that easily condense into liquid and sometimes interfere with
or mask the water dew point. When this occurs, it is sometimes very
the existing pressure.
helpful to supplement the apparatus in Fig. 1 with an optical attachment
that uniformly illuminates the dew–point mirror and also magnifies the
3. Significance and Use
condensate on the mirror. With this attachment it is possible, in some
cases, to observe separate condensation points of water vapor,
3.1 Generally,contractsgoverningthepipelinetransmission
hydrocarbons,andglycolaminesaswellasicepoints.However,ifthedew
of natural gas contain specifications limiting the maximum
point of the condensable hydrocarbons is higher than the water vapor dew
concentration of water vapor allowed. Excess water vapor can
point, when such hydrocarbons are present in large amounts, they may
flood the mirror and obscure or wash off the water dew point. Best results causecorrosiveconditions,degradingpipelinesandequipment.
in distinguishing multiple component dew points are obtained when they
It can also condense and freeze or form methane hydrates
are not too closely spaced.
causing blockages. Water–vapor content also affects the heat-
NOTE 2—Condensation of water vapor on the dew-point mirror may
ingvalueofnaturalgas,thusinfluencingthequalityofthegas.
appear as liquid water at temperatures as low as 0 to−10°F (−18
This test method permits the determination of water content of
to−23°C). At lower temperatures an ice point rather than a water dew
point likely will be observed. The minimum dew point of any vapor that natural gas.
can be observed is limited by the mechanical parts of the equipment.
Mirror temperatures as low as−150°F (−100°C) have been measured,
4. Apparatus
using liquid nitrogen as the coolant with a thermocouple attached to the
mirror, instead of a thermometer well.
4.1 Any properly constructed dew-point apparatus may be
1.2 This standard does not purport to address all of the used that satisfies the basic requirements that means must be
provided:
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro- 4.1.1 To permit a controlled flow of gas to enter and leave
priate safety and health practices and determine the applica- the apparatus while the apparatus is at a temperature at least
bility of regulatory limitations prior to use. 3°F above the dew point of the gas.
4.1.2 To cool and control the cooling rate of a portion
2. Terminology
(preferably a small portion) of the apparatus, with which the
flowing gas comes in contact, to a temperature low enough to
2.1 Definitions of Terms Specific to This Standard:
cause vapor to condense from the gas.
2.1.1 saturated water vapor or equilibrium water–vapor
4.1.3 To observe the deposition of dew on the cold portion
content—the water vapor concentration in a gas mixture that is
of the apparatus.
in equilibrium with a liquid phase of pure water that is
4.1.4 To measure the temperature of the cold portion on the
apparatus on which the dew is deposited, and
1
4.1.5 To measure the pressure of the gas within the appara-
ThistestmethodisunderthejurisdictionofASTMCommitteeD03onGaseous
Fuels and is the direct responsibility of Subcommittee D03.05 on Determination of
tus or the deviation from the known existing barometric
Special Constituents of Gaseous Fuels.
pressure.
Current edition approved Nov. 1, 2012. Published December 2012. Originally
4.1.6 The apparatus should be constructed so that the “cold
approved in 1950. Last previous edition approved in 2006 as D1142–95(2006).
DOI: 10.1520/D1142-95R12.
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.